The paroxysmal depolarization shift in epilepsy research
- PMID: 30557621
- PMCID: PMC7116775
- DOI: 10.1016/j.biocel.2018.12.006
The paroxysmal depolarization shift in epilepsy research
Abstract
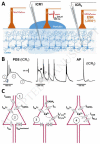
Several shortcomings with currently available pharmacotherapy of epilepsy necessitate the search for new drug targets. Paroxysmal depolarization shifts (PDS) represent the cellular correlates of electrographic (e.g. interictal) spikes. While the ionic basis of PDS is understood in great detail, controversy exists regarding their proposed implication in epilepsy. To address this issue and to consider potential targetability, this mini-review gives an overview of the ionic conductances contributing to PDS and reflects on the hypotheses of their potential pro-epileptic (epileptogenic) and anti-epileptic roles.
Keywords: Epileptogenesis; Hippocampal neurons; Interictal spike; L-type voltage-gated calcium channels.
Copyright © 2018 Elsevier Ltd. All rights reserved.
Figures
Similar articles
-
The Paroxysmal Depolarization Shift: Reconsidering Its Role in Epilepsy, Epileptogenesis and Beyond.Int J Mol Sci. 2019 Jan 29;20(3):577. doi: 10.3390/ijms20030577. Int J Mol Sci. 2019. PMID: 30699993 Free PMC article. Review.
-
On the Origin of Paroxysmal Depolarization Shifts: The Contribution of Cav1.x Channels as the Common Denominator of a Polymorphous Neuronal Discharge Pattern.Neuroscience. 2021 Aug 1;468:265-281. doi: 10.1016/j.neuroscience.2021.05.011. Epub 2021 May 18. Neuroscience. 2021. PMID: 34015369
-
Cav 1.3 channels play a crucial role in the formation of paroxysmal depolarization shifts in cultured hippocampal neurons.Epilepsia. 2017 May;58(5):858-871. doi: 10.1111/epi.13719. Epub 2017 Mar 11. Epilepsia. 2017. PMID: 28295232 Free PMC article.
-
Interictal spikes: harbingers or causes of epilepsy?Neurosci Lett. 2011 Jun 27;497(3):247-50. doi: 10.1016/j.neulet.2011.03.070. Epub 2011 Mar 31. Neurosci Lett. 2011. PMID: 21458535 Free PMC article. Review.
-
The Paroxysmal Depolarizing Shift: The First Cellular Marker of Focal Epileptogenesis.In: Noebels JL, Avoli M, Rogawski MA, Vezzani A, Delgado-Escueta AV, editors. Jasper's Basic Mechanisms of the Epilepsies. 5th edition. New York: Oxford University Press; 2024. Chapter 1. In: Noebels JL, Avoli M, Rogawski MA, Vezzani A, Delgado-Escueta AV, editors. Jasper's Basic Mechanisms of the Epilepsies. 5th edition. New York: Oxford University Press; 2024. Chapter 1. PMID: 39637141 Free Books & Documents. Review.
Cited by
-
Early hippocampal high-amplitude rhythmic spikes predict post-traumatic epilepsy in mice.Front Neurosci. 2024 Aug 29;18:1422449. doi: 10.3389/fnins.2024.1422449. eCollection 2024. Front Neurosci. 2024. PMID: 39268032 Free PMC article.
-
Noradrenergic stimulation of α1 adrenoceptors in the medial prefrontal cortex mediates acute stress-induced facilitation of seizures in mice.Sci Rep. 2023 May 19;13(1):8089. doi: 10.1038/s41598-023-35242-0. Sci Rep. 2023. PMID: 37208473 Free PMC article.
-
Of the Mechanisms of Paroxysmal Depolarization Shifts: Generation and Maintenance of Bicuculline-Induced Paroxysmal Activity in Rat Hippocampal Cell Cultures.Int J Mol Sci. 2023 Jul 1;24(13):10991. doi: 10.3390/ijms241310991. Int J Mol Sci. 2023. PMID: 37446169 Free PMC article.
-
The Paroxysmal Depolarization Shift: Reconsidering Its Role in Epilepsy, Epileptogenesis and Beyond.Int J Mol Sci. 2019 Jan 29;20(3):577. doi: 10.3390/ijms20030577. Int J Mol Sci. 2019. PMID: 30699993 Free PMC article. Review.
-
Actions of the antiseizure drug carbamazepine in the thalamic reticular nucleus: Potential mechanism of aggravating absence seizures.Proc Natl Acad Sci U S A. 2025 Aug 5;122(31):e2500644122. doi: 10.1073/pnas.2500644122. Epub 2025 Jul 31. Proc Natl Acad Sci U S A. 2025. PMID: 40743388
References
-
- Akaishi T, Nakazawa K, Sato K, Saito H, Ohno Y, Ito Y. Hydrogen peroxide modulates whole cell Ca2+ currents through L-type channels in cultured rat dentate granule cells. Neurosci Lett. 2004;356:25–28. - PubMed
-
- Antoniadis A, Müller WE, Wollert U. Inhibition of GABA and benzodiazepine receptor binding by penicillins. Neurosci Lett. 1980;18:309–312. - PubMed
-
- Bingmann D, Speckmann EJ, Baker RE, Ruijter J, de Jong BM. Differential antiepileptic effects of the organic calcium antagonists verapamil and flunarizine in neurons of organotypic neocortical explants from newborn rats. Exp Brain Res. 1988;72:439–442. - PubMed
-
- Chen S, Su H, Yue C, Remy S, Royeck M, Sochivko D, Opitz T, Beck H, Yaari Y. An increase in persistent sodium current contributes to intrinsic neuronal bursting after status epilepticus. J Neurophysiol. 2011;105:117–129. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

