EDUCATIONAL SERIES IN CONGENITAL HEART DISEASE: Tetralogy of Fallot: diagnosis to long-term follow-up
- PMID: 30557849
- PMCID: PMC6301192
- DOI: 10.1530/ERP-18-0049
EDUCATIONAL SERIES IN CONGENITAL HEART DISEASE: Tetralogy of Fallot: diagnosis to long-term follow-up
Abstract
Tetralogy of Fallot (TOF) is the most common cyanotic congenital heart defect, affecting 3 in 10,000 live births. Surgical correction in early childhood is associated with good outcomes, but lifelong follow-up is necessary to identify the long-term sequelae that may occur. This article will cover the diagnosis of TOF in childhood, the objectives of surveillance through adulthood and the value of multi-modality imaging in identifying and guiding timely surgical and percutaneous interventions.
Keywords: congenital heart disease; multi-modality imaging; pulmonary regurgitation; tetralogy of Fallot.
Figures
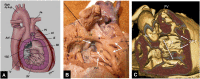
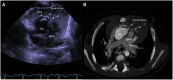
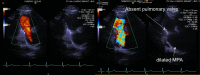
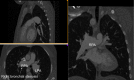


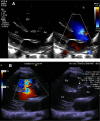
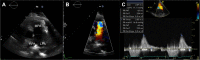
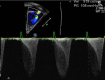

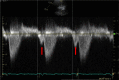
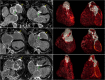
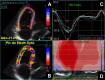
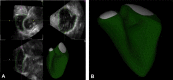
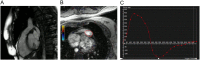
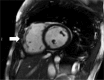
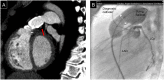
References
-
- Suzuki A, Ho SY, Anderson RH, Deanfield JE. Further morphologic studies on tetralogy of Fallot, with particular emphasis on the prevalence and structure of the membranous flap. Journal of Thoracic and Cardiovascular Surgery 1990. 99 528–535. - PubMed
-
- Ramaswamy P, Lytrivi ID, Thanjan MT, Nguyen T, Srivastava S, Sharma S, Ko HH, Parness IA, Lai WW. Frequency of aberrant subclavian artery, arch laterality, and associated intracardiac anomalies detected by echocardiography. American Journal of Cardiology 2008. 101 677–682. (10.1016/j.amjcard.2007.10.036) - DOI - PubMed
-
- Nakata S, Imai Y, Takanashi Y, Kurosawa H, Tezuka K, Nakazawa M, Ando M, Takao A. A new method for the quantitative standardization of cross-sectional areas of the pulmonary arteries in congenital heart diseases with decreased pulmonary blood flow. Journal of Thoracic and Cardiovascular Surgery 1984. 88 610–619. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

