Compatibility and stability of dezocine and tropisetron in 0.9% sodium chloride injection for patient-controlled analgesia administration
- PMID: 30558082
- PMCID: PMC6319974
- DOI: 10.1097/MD.0000000000013698
Compatibility and stability of dezocine and tropisetron in 0.9% sodium chloride injection for patient-controlled analgesia administration
Abstract
Tropisetron is an adjuvant for dezocine used in intravenous patient-controlled analgesia (PCA) and has been reported to provide superior pain control. It is efficacious in reducing the institutional incidence of postoperative nausea and vomiting (PONV), which decreases resource utilization and cost. However, no scientific evidence has been reported in the literature demonstrating analytical confirmation of the compatibility and stability of the combination of dezocine and tropisetron. Thus, the present study aimed to investigate the stability of dezocine with tropisetron in 0.9% sodium chloride injection form for PCA administration.Commercial solutions of dezocine and tropisetron were combined and examined for compatibility and stability when diluted with 0.9% sodium chloride injection in polyolefin bags and glass bottles stored at 4°C or 25°C for up to 14 days. The initial concentrations were 40 mg/100 mL dezocine and 5 mg/100 mL tropisetron. For all samples, the compatibility parameters (including precipitation, cloudiness, discoloration, and pH values) were evaluated. Chemical stability was also determined using high-performance liquid chromatographic (HPLC) analysis.After a 14-day period of storage at 4°C or 25°C, the initial concentrations of dezocine and tropisetron were maintained at at least 98%. All of the mixtures remained clear and colorless throughout the observation period, and no color change or precipitation was observed.These results indicated that admixtures of 40 mg/100 mL dezocine and 5 mg/100 mL tropisetron in 0.9% sodium chloride injection were stable for at least 14 days when stored in polyolefin bags or glass bottles at 4°C or 25°C and protected from light.
Conflict of interest statement
The authors have no conflicts of interest to disclose.
Figures
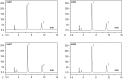

Similar articles
-
Stability study of dezocine in 0.9% sodium chloride solutions for patient-controlled analgesia administration.Medicine (Baltimore). 2017 Sep;96(35):e7979. doi: 10.1097/MD.0000000000007979. Medicine (Baltimore). 2017. PMID: 28858135 Free PMC article.
-
Physical compatibility and chemical stability of dezocine and ramosetron in 0.9% sodium chloride injection for patient-controlled analgesia administration.Medicine (Baltimore). 2022 Nov 11;101(45):e31546. doi: 10.1097/MD.0000000000031546. Medicine (Baltimore). 2022. PMID: 36397408 Free PMC article.
-
Stability of butorphanol-tropisetron mixtures in 0.9% sodium chloride injection for patient-controlled analgesia use.Medicine (Baltimore). 2015 Feb;94(6):e432. doi: 10.1097/MD.0000000000000432. Medicine (Baltimore). 2015. PMID: 25674732 Free PMC article.
-
Dezocine as a potent analgesic: overview of its pharmacological characterization.Acta Pharmacol Sin. 2022 Jul;43(7):1646-1657. doi: 10.1038/s41401-021-00790-6. Epub 2021 Nov 4. Acta Pharmacol Sin. 2022. PMID: 34737418 Free PMC article. Review.
-
Analgesic comparison of dezocine plus propofol versus fentanyl plus propofol for gastrointestinal endoscopy: A meta-analysis.Medicine (Baltimore). 2021 Apr 16;100(15):e25531. doi: 10.1097/MD.0000000000025531. Medicine (Baltimore). 2021. PMID: 33847679 Free PMC article.
Cited by
-
A Fast and Validated HPLC Method for the Simultaneous Analysis of Five 5-HT3 Receptor Antagonists via the Quantitative Analysis of Multicomponents by a Single Marker.Int J Anal Chem. 2021 Jun 26;2021:5533646. doi: 10.1155/2021/5533646. eCollection 2021. Int J Anal Chem. 2021. PMID: 34257662 Free PMC article.
-
Stability evaluation of morphine, hydromorphone, metamizole and esketamine containing analgesic mixtures applied for patient-controlled analgesia in hospice and palliative care.Biomed Chromatogr. 2022 Apr;36(4):e5340. doi: 10.1002/bmc.5340. Epub 2022 Jan 26. Biomed Chromatogr. 2022. PMID: 35043434 Free PMC article.
-
Stability and Compatibility of Ramosetron with Midazolam in 0.9% Sodium Chloride Injection for Postoperative Nausea and Vomiting Administration.Drug Des Devel Ther. 2020 Mar 17;14:1169-1176. doi: 10.2147/DDDT.S244439. eCollection 2020. Drug Des Devel Ther. 2020. PMID: 32256048 Free PMC article.
References
-
- Pasero C, Quinlancolwell A, Rae D, et al. American Society for Pain Management Nursing Position Statement: prescribing and administering opioid doses based solely on pain intensity. Pain Manag Nurs 2016;17:170–80. - PubMed
-
- Ren BX, Zong J, Tang JC, et al. Effects of intravenous analgesia with combined dezocine and butorphanol on postoperative cognitive function in elderly patients. Genet Mol Res 2015;14:5571–6. - PubMed
-
- Li S, Min S, Wu B, et al. Application of patient-controlled intravenous analgesia of dezocine combined with sufentanil in burn patients after surgery. Zhonghua Shao Shang Za Zhi 2015;31:48–51. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

