Compatibility and stability of dezocine and tropisetron in 0.9% sodium chloride injection for patient-controlled analgesia administration
- PMID: 30558082
- PMCID: PMC6319974
- DOI: 10.1097/MD.0000000000013698
Compatibility and stability of dezocine and tropisetron in 0.9% sodium chloride injection for patient-controlled analgesia administration
Abstract
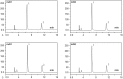
Tropisetron is an adjuvant for dezocine used in intravenous patient-controlled analgesia (PCA) and has been reported to provide superior pain control. It is efficacious in reducing the institutional incidence of postoperative nausea and vomiting (PONV), which decreases resource utilization and cost. However, no scientific evidence has been reported in the literature demonstrating analytical confirmation of the compatibility and stability of the combination of dezocine and tropisetron. Thus, the present study aimed to investigate the stability of dezocine with tropisetron in 0.9% sodium chloride injection form for PCA administration.Commercial solutions of dezocine and tropisetron were combined and examined for compatibility and stability when diluted with 0.9% sodium chloride injection in polyolefin bags and glass bottles stored at 4°C or 25°C for up to 14 days. The initial concentrations were 40 mg/100 mL dezocine and 5 mg/100 mL tropisetron. For all samples, the compatibility parameters (including precipitation, cloudiness, discoloration, and pH values) were evaluated. Chemical stability was also determined using high-performance liquid chromatographic (HPLC) analysis.After a 14-day period of storage at 4°C or 25°C, the initial concentrations of dezocine and tropisetron were maintained at at least 98%. All of the mixtures remained clear and colorless throughout the observation period, and no color change or precipitation was observed.These results indicated that admixtures of 40 mg/100 mL dezocine and 5 mg/100 mL tropisetron in 0.9% sodium chloride injection were stable for at least 14 days when stored in polyolefin bags or glass bottles at 4°C or 25°C and protected from light.
Conflict of interest statement
The authors have no conflicts of interest to disclose.
Figures

References
-
- Pasero C, Quinlancolwell A, Rae D, et al. American Society for Pain Management Nursing Position Statement: prescribing and administering opioid doses based solely on pain intensity. Pain Manag Nurs 2016;17:170–80. - PubMed
-
- Ren BX, Zong J, Tang JC, et al. Effects of intravenous analgesia with combined dezocine and butorphanol on postoperative cognitive function in elderly patients. Genet Mol Res 2015;14:5571–6. - PubMed
-
- Li S, Min S, Wu B, et al. Application of patient-controlled intravenous analgesia of dezocine combined with sufentanil in burn patients after surgery. Zhonghua Shao Shang Za Zhi 2015;31:48–51. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

