Agrimonia procera Wallr. Extract Increases Stress Resistance and Prolongs Life Span in Caenorhabditis elegans via Transcription Factor DAF-16 (FoxO Orthologue)
- PMID: 30558122
- PMCID: PMC6315603
- DOI: 10.3390/antiox7120192
Agrimonia procera Wallr. Extract Increases Stress Resistance and Prolongs Life Span in Caenorhabditis elegans via Transcription Factor DAF-16 (FoxO Orthologue)
Abstract
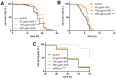
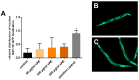
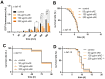
Agrimonia procera is a pharmacologically interesting plant which is proposed to protect against various diseases due to its high amount of phytochemicals, e.g., polyphenols. However, in spite of the amount of postulated health benefits, studies concerning the mechanistic effects of Agrimonia procera are limited. Using the nematode Caenorhabditis elegans, we were able to show that an ethanol extract of Agrimonia procera herba (eAE) mediates strong antioxidative effects in the nematode: Beside a strong radical-scavenging activity, eAE reduces accumulation of reactive oxygen species (ROS) accumulation and protects against paraquat-induced oxidative stress. The extract does not protect against amyloid-β-mediated toxicity, but efficiently increases the life span (up to 12.7%), as well as the resistance to thermal stress (prolongation of survival up to 22%), of this model organism. Using nematodes deficient in the forkhead box O (FoxO)-orthologue DAF-16, we were able to demonstrate that beneficial effects of eAE on stress resistance and life span were mediated via this transcription factor. We showed antioxidative, stress-reducing, and life-prolonging effects of eAE in vivo and were able to demonstrate a molecular mechanism of this extract. These results may be important for identifying further molecular targets of eAE in humans.
Keywords: aging; antioxidant; insulin-like signaling; life span-extending effects; oxidative stress; phytochemicals.
Conflict of interest statement
G.H. is managing director of Exsemine GmbH providing the plant material. He has no influence on the conduction/analysis of the experiments. The other authors declare no conflict of interest.
Figures

References
-
- Granica S., Kluge H., Horn G., Matkowski A., Kiss A.K. The phytochemical investigation of Agrimonia eupatoria L. and Agrimonia procera Wallr. as valid sources of Agrimoniae herba. The pharmacopoeial plant material. J. Pharm. Biomed. Anal. 2015;114:272–279. doi: 10.1016/j.jpba.2015.05.027. - DOI - PubMed
-
- Kiraly G., Kiraly A. Agrimonia procera Wallr. in Hungary–distribution and habitat characteristics. Flora Pannon. 2004;2:7–24.
-
- Pukalskienė M., Slapšytė G., Dedonytė V., Lazutka J.R., Mierauskienė J., Venskutonis P.R. Genotoxicity and antioxidant activity of five Agrimonia and Filipendula species plant extracts evaluated by comet and micronucleus assays in human lymphocytes and Ames Salmonella/microsome test. Food Chem. Toxicol. 2018;113:303–313. doi: 10.1016/j.fct.2017.12.031. - DOI - PubMed
-
- Gräber T., Kluge H., Granica S., Horn G., Kalbitz J., Brandsch C., Breitenstein A., Brütting C., Stangl G.I. Agrimonia procera exerts antimicrobial effects, modulates the expression of defensins and cytokines in colonocytes and increases the immune response in lipopolysaccharide-challenged piglets. BMC Vet. Res. 2018;14:346. doi: 10.1186/s12917-018-1680-0. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

