Schisandra Chinensis Lignans Suppresses the Production of Inflammatory Mediators Regulated by NF-κB, AP-1, and IRF3 in Lipopolysaccharide-Stimulated RAW264.7 Cells
- PMID: 30558163
- PMCID: PMC6320760
- DOI: 10.3390/molecules23123319
Schisandra Chinensis Lignans Suppresses the Production of Inflammatory Mediators Regulated by NF-κB, AP-1, and IRF3 in Lipopolysaccharide-Stimulated RAW264.7 Cells
Abstract
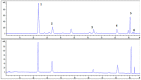
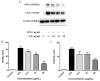
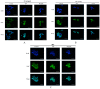
Schisandra Fructus (SF) is a traditional Chinese herb used in the treatment of inflammatory disorders like hepatitis. One of the main anti-inflammatory components of SF is the lignans. However, the underlying anti-inflammatory mechanism of Schisandra Chinensis lignans (SCL) remains unclear. This study aims to investigate the effects of SCL on inflammatory mediators in lipopolysaccharide-stimulated RAW264.7 cells and explore the underlying mechanism. The production of nitric oxide (NO) was determined by Griess reaction. ELISA was used to determine cytokine levels and chemokines secretion. To estimate protein levels and enzyme activities, we employed Western blotting. Nuclear localization of NF-κB, AP-1, and IRF3 was detected using immunofluorescence analyses. The results showed that SCL significantly reduced the release of inflammatory mediators, including NO and PGE2, which may be related to down-regulation of iNOS and COX-2 expression. The production of cytokines and chemokines was suppressed by SCL treatment. SCL also decreased the phosphorylation of IKKα/β, IκB-α, Akt, TBK1, ERK, p38, JNK, NF-κB (p65), AP-1 (c-Jun), and IRF3 in RAW264.7 macrophages activated with LPS. The nuclear protein levels and nuclear translocation of AP-1, NF-κB and IRF3 were suppressed by SCL. These results indicated that SCL suppressed the IKKα/β/NF-κB, MAPKs/AP-1 and TBK1/IRF3 signaling pathways in LPS-stimulated RAW264.7 macrophages.
Keywords: AP-1; IRF3; NF-κB; RAW264.7 macrophages; Schisandra Chinensis lignans; anti-inflammation.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures






References
-
- Montón C., Torres A. Lung inflammatory response in pneumonia. Monaldi Arch. Chest Dis. 1998;53:56–63. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

