Proteomic Analysis of Zn Depletion/Repletion in the Hormone-Secreting Thyroid Follicular Cell Line FRTL-5
- PMID: 30558183
- PMCID: PMC6315927
- DOI: 10.3390/nu10121981
Proteomic Analysis of Zn Depletion/Repletion in the Hormone-Secreting Thyroid Follicular Cell Line FRTL-5
Abstract
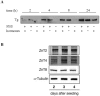
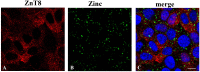
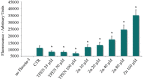
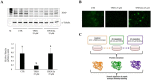
Zinc deficiency predisposes to a wide spectrum of chronic diseases. The human Zn proteome was predicted to represent about 10% of the total human proteome, reflecting the broad array of metabolic functions in which this micronutrient is known to participate. In the thyroid, Zn was reported to regulate cellular homeostasis, with a yet elusive mechanism. The Fischer Rat Thyroid Cell Line FRTL-5 cell model, derived from a Fischer rat thyroid and displaying a follicular cell phenotype, was used to investigate a possible causal relationship between intracellular Zn levels and thyroid function. A proteomic approach was applied to compare proteins expressed in Zn deficiency, obtained by treating cells with the Zn-specific chelator N,N,N',N'-tetrakis (2-pyridylmethyl) ethylene-diamine (TPEN), with Zn repleted cells. Quantitative proteomic analysis of whole cell protein extracts was performed using stable isotope dimethyl labelling coupled to nano-ultra performance liquid chromatography-mass spectrometry (UPLC-MS). TPEN treatment led to almost undetectable intracellular Zn, while decreasing thyroglobulin secretion. Subsequent addition of ZnSO₄ fully reversed these phenotypes. Comparative proteomic analysis of Zn depleted/repleted cells identified 108 proteins modulated by either treatment. Biological process enrichment analysis identified functions involved in calcium release and the regulation of translation as the most strongly regulated processes in Zn depleted cells.
Keywords: calcium channels; endocrine tissues; metal ion; ribosomes; zinc transport.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Influence of intracellular zinc on cultures of rat cardiac neural crest cells.Birth Defects Res B Dev Reprod Toxicol. 2015 Feb;104(1):11-22. doi: 10.1002/bdrb.21135. Epub 2015 Feb 16. Birth Defects Res B Dev Reprod Toxicol. 2015. PMID: 25689142
-
DNA methylation mechanism of intracellular zinc deficiency-induced injury in primary hippocampal neurons in the rat brain.Nutr Neurosci. 2018 Sep;21(7):478-486. doi: 10.1080/1028415X.2017.1312090. Epub 2017 Apr 19. Nutr Neurosci. 2018. PMID: 28421879
-
Complex role of zinc in methamphetamine toxicity in vitro.Neuroscience. 2010 Nov 24;171(1):31-9. doi: 10.1016/j.neuroscience.2010.08.049. Epub 2010 Aug 27. Neuroscience. 2010. PMID: 20801194 Free PMC article.
-
Proteomic High Affinity Zn2+ Trafficking: Where Does Metallothionein Fit in?Int J Mol Sci. 2017 Jun 17;18(6):1289. doi: 10.3390/ijms18061289. Int J Mol Sci. 2017. PMID: 28629147 Free PMC article. Review.
-
The role of zinc in the prevention of diabetic cardiomyopathy and nephropathy.Toxicol Mech Methods. 2013 Jan;23(1):27-33. doi: 10.3109/15376516.2012.735277. Epub 2012 Nov 29. Toxicol Mech Methods. 2013. PMID: 23039870 Review.
Cited by
-
Zinc as a countermeasure for cadmium toxicity.Acta Pharmacol Sin. 2021 Mar;42(3):340-346. doi: 10.1038/s41401-020-0396-4. Epub 2020 Apr 13. Acta Pharmacol Sin. 2021. PMID: 32284539 Free PMC article. Review.
-
Nutraceuticals in Thyroidology: A Review of in Vitro, and in Vivo Animal Studies.Nutrients. 2020 May 8;12(5):1337. doi: 10.3390/nu12051337. Nutrients. 2020. PMID: 32397091 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

