Cardioprotective Effects of Puerarin-V on Isoproterenol-Induced Myocardial Infarction Mice Is Associated with Regulation of PPAR-Υ/NF-κB Pathway
- PMID: 30558188
- PMCID: PMC6321361
- DOI: 10.3390/molecules23123322
Cardioprotective Effects of Puerarin-V on Isoproterenol-Induced Myocardial Infarction Mice Is Associated with Regulation of PPAR-Υ/NF-κB Pathway
Abstract
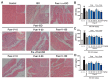
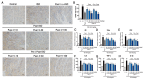
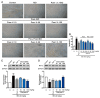
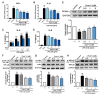
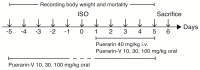
Puerarin is a well-known traditional Chinese medicine which has been used for the treatment of cardiovascular diseases. Recently, a new advantageous crystal form of puerarin, puerarin-V, has been developed. However, the cardioprotective effects of puerarin-V on myocardial infarction (MI) heart failure are still unclear. In this research, we aim to evaluate the cardioprotective effects of puerarin-V on the isoproterenol (ISO)-induced MI mice and elucidate the underlying mechanisms. To induce MI in C57BL/6 mice, ISO was administered at 40 mg/kg subcutaneously every 12 h for three times in total. The mice were randomly divided into nine groups: (1) control; (2) ISO; (3) ISO + puerarin injection; (4⁻9) ISO + puerarin-V at different doses and timings. After treatment, cardiac function was evaluated by electrocardiogram (ECG), biochemical and histochemical analysis. In vitro inflammatory responses and apoptosis were evaluated in human coronary artery endothelial cells (HCAECs) challenged by lipopolysaccharide (LPS). LPS-induced PPAR-Υ/NF-κB and subsequently activation of cytokines were assessed by the western blot and real-time polymerase chain reaction (PCR). Administration of puerarin-V significantly inhibits the typical ST segment depression compared with that in MI mice. Further, puerarin-V treatment significantly improves ventricular wall infarction, decreases the incidence of mortality, and inhibits the levels of myocardial injury markers. Moreover, puerarin-V treatment reduces the inflammatory milieu in the heart of MI mice, thereby blocking the upregulation of proinflammatory cytokines (TNF-α, IL-1β and IL-6). The beneficial effects of puerarin-V might be associated with the normalization in gene expression of PPAR-Υ and PPAR-Υ/NF-κB /ΙκB-α/ΙΚΚα/β phosphorylation. In the in vitro experiment, treatment with puerarin-V (0.3, 1 and 3 μM) significantly reduces cell death and suppresses the inflammation cytokines expression. Likewise, puerarin-V exhibits similar mechanisms. The cardioprotective effects of puerarin-V treatment on MI mice in the pre + post-ISO group seem to be more prominent compared to those in the post-ISO group. Puerarin-V exerts cardioprotective effects against ISO-induced MI in mice, which may be related to the activation of PPAR-γ and the inhibition of NF-κB signaling in vivo and in vitro. Taken together, our research provides a new therapeutic option for the treatment of MI in clinic.
Keywords: NF-κB; PPAR-Υ; anti-apoptosis; anti-inflammation; myocardial infarction; puerarin-V.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




Similar articles
-
Green Synthesis and Chemical Characterization of Silver Nanoparticles by Tribulus terrestris for the Treatment of Myocardial Infarction.Biol Trace Elem Res. 2025 Apr;203(4):1961-1971. doi: 10.1007/s12011-024-04336-9. Epub 2024 Aug 12. Biol Trace Elem Res. 2025. PMID: 39134771
-
Puerarin prevents vascular endothelial injury through suppression of NF-κB activation in LPS-challenged human umbilical vein endothelial cells.Biomed Pharmacother. 2018 Aug;104:261-267. doi: 10.1016/j.biopha.2018.05.038. Epub 2018 May 25. Biomed Pharmacother. 2018. PMID: 29775893
-
Puerarin prevents LPS-induced acute lung injury via inhibiting inflammatory response.Microb Pathog. 2018 May;118:170-176. doi: 10.1016/j.micpath.2018.03.033. Epub 2018 Mar 20. Microb Pathog. 2018. PMID: 29571724
-
Puerarin: A review of its mechanisms of action and clinical studies in ophthalmology.Phytomedicine. 2022 Dec;107:154465. doi: 10.1016/j.phymed.2022.154465. Epub 2022 Sep 19. Phytomedicine. 2022. PMID: 36166943 Review.
-
Role of puerarin in pathological cardiac remodeling: A review.Pharmacol Res. 2022 Apr;178:106152. doi: 10.1016/j.phrs.2022.106152. Epub 2022 Mar 4. Pharmacol Res. 2022. PMID: 35248700 Review.
Cited by
-
Laurel Attenuates Dexamethasone-Induced Skeletal Muscle Atrophy In Vitro and in a Rat Model.Nutrients. 2022 May 12;14(10):2029. doi: 10.3390/nu14102029. Nutrients. 2022. PMID: 35631169 Free PMC article.
-
The Role of Ferroptosis in Cardiovascular Disease and Its Therapeutic Significance.Front Cardiovasc Med. 2021 Oct 26;8:733229. doi: 10.3389/fcvm.2021.733229. eCollection 2021. Front Cardiovasc Med. 2021. PMID: 34765653 Free PMC article. Review.
-
Abatacept: A Promising Repurposed Solution for Myocardial Infarction-Induced Inflammation in Rat Models.Oxid Med Cell Longev. 2024 Mar 13;2024:3534104. doi: 10.1155/2024/3534104. eCollection 2024. Oxid Med Cell Longev. 2024. PMID: 38957586 Free PMC article.
-
Exercise Mediated Nrf2 Signaling Protects the Myocardium From Isoproterenol-Induced Pathological Remodeling.Front Cardiovasc Med. 2019 Jun 6;6:68. doi: 10.3389/fcvm.2019.00068. eCollection 2019. Front Cardiovasc Med. 2019. PMID: 31245386 Free PMC article.
-
Tilianin suppresses NLRP3 inflammasome activation in myocardial ischemia/reperfusion injury via inhibition of TLR4/NF-κB and NEK7/NLRP3.Front Pharmacol. 2024 Oct 23;15:1423053. doi: 10.3389/fphar.2024.1423053. eCollection 2024. Front Pharmacol. 2024. PMID: 39508038 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

