The Complex Interplay between Lipids, Immune System and Interleukins in Cardio-Metabolic Diseases
- PMID: 30558209
- PMCID: PMC6321433
- DOI: 10.3390/ijms19124058
The Complex Interplay between Lipids, Immune System and Interleukins in Cardio-Metabolic Diseases
Abstract
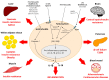
Lipids and inflammation regulate each other. Early studies on this topic focused on the systemic effects that the acute inflammatory response-and interleukins-had on lipid metabolism. Today, in the era of the obesity epidemic, whose primary complications are cardio-metabolic diseases, attention has moved to the effects that the nutritional environment and lipid derangements have on peripheral tissues, where lipotoxicity leads to organ damage through an imbalance of chronic inflammatory responses. After an overview of the effects that acute inflammation has on the systemic lipid metabolism, this review will describe the lipid-induced immune responses that take place in peripheral tissues and lead to chronic cardio-metabolic diseases. Moreover, the anti-inflammatory effects of lipid lowering drugs, as well as the possibility of using anti-inflammatory agents against cardio-metabolic diseases, will be discussed.
Keywords: cholesterol; free fatty acids; innate immune system; interleukin; lipid; lipotoxicity; triglyceride.
Conflict of interest statement
The authors declare that there are no conflicts of interest associated with the manuscript.
Figures

Similar articles
-
Role of interleukins in obesity: implications for metabolic disease.Trends Endocrinol Metab. 2014 Jun;25(6):312-9. doi: 10.1016/j.tem.2014.02.004. Epub 2014 Mar 31. Trends Endocrinol Metab. 2014. PMID: 24698032 Review.
-
Bile Acids in the Treatment of Cardiometabolic Diseases.Ann Hepatol. 2017 Nov;16(Suppl. 1: s3-105.):s43-s52. doi: 10.5604/01.3001.0010.5496. Ann Hepatol. 2017. PMID: 29080341 Review.
-
Interactions between inflammation and lipid metabolism: relevance for efficacy of anti-inflammatory drugs in the treatment of atherosclerosis.Atherosclerosis. 2013 Jun;228(2):306-15. doi: 10.1016/j.atherosclerosis.2013.02.028. Epub 2013 Mar 1. Atherosclerosis. 2013. PMID: 23518178 Review.
-
Interleukin-22 alleviates metabolic disorders and restores mucosal immunity in diabetes.Nature. 2014 Oct 9;514(7521):237-41. doi: 10.1038/nature13564. Epub 2014 Aug 6. Nature. 2014. PMID: 25119041
-
The cellular and signaling networks linking the immune system and metabolism in disease.Nat Med. 2012 Mar 6;18(3):363-74. doi: 10.1038/nm.2627. Nat Med. 2012. PMID: 22395709 Review.
Cited by
-
The multifaceted role of PCSK9 in cancer pathogenesis, tumor immunity, and immunotherapy.Med Oncol. 2024 Jul 15;41(8):202. doi: 10.1007/s12032-024-02435-0. Med Oncol. 2024. PMID: 39008137 Review.
-
The Role of ABC Transporters in Lipid Metabolism and the Comorbid Course of Chronic Obstructive Pulmonary Disease and Atherosclerosis.Int J Mol Sci. 2021 Jun 23;22(13):6711. doi: 10.3390/ijms22136711. Int J Mol Sci. 2021. PMID: 34201488 Free PMC article. Review.
-
Association of Advanced Lipoprotein Subpopulation Profiles with Insulin Resistance and Inflammation in Patients with Type 2 Diabetes Mellitus.J Clin Med. 2023 Jan 6;12(2):487. doi: 10.3390/jcm12020487. J Clin Med. 2023. PMID: 36675414 Free PMC article.
-
Expanding the therapeutic landscape: ezetimibe as non-statin therapy for dyslipidemia.Korean J Intern Med. 2023 Nov;38(6):797-809. doi: 10.3904/kjim.2023.243. Epub 2023 Oct 20. Korean J Intern Med. 2023. PMID: 37866817 Free PMC article. Review.
-
Molecular Mechanisms of Lipid Metabolism Disorders in Infectious Exacerbations of Chronic Obstructive Pulmonary Disease.Int J Mol Sci. 2021 Jul 17;22(14):7634. doi: 10.3390/ijms22147634. Int J Mol Sci. 2021. PMID: 34299266 Free PMC article. Review.
References
-
- Akdis M., Aab A., Altunbulakli C., Azkur K., Costa R.A., Crameri R., Duan S., Eiwegger T., Eljaszewicz A., Ferstl R., et al. Interleukins (from IL-1 to IL-38), interferons, transforming growth factor beta, and TNF-alpha: Receptors, functions, and roles in diseases. J. Allergy Clin. Immunol. 2016;138:984–1010. doi: 10.1016/j.jaci.2016.06.033. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

