Targeting the Oncoprotein Smoothened by Small Molecules: Focus on Novel Acylguanidine Derivatives as Potent Smoothened Inhibitors
- PMID: 30558232
- PMCID: PMC6316656
- DOI: 10.3390/cells7120272
Targeting the Oncoprotein Smoothened by Small Molecules: Focus on Novel Acylguanidine Derivatives as Potent Smoothened Inhibitors
Abstract
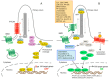
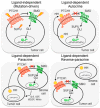
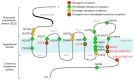
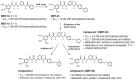
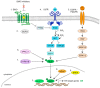
Hedgehog-GLI (HH) signaling was originally identified as a critical morphogenetic pathway in embryonic development. Since its discovery, a multitude of studies have reported that HH signaling also plays key roles in a variety of cancer types and in maintaining tumor-initiating cells. Smoothened (SMO) is the main transducer of HH signaling, and in the last few years, it has emerged as a promising therapeutic target for anticancer therapy. Although vismodegib and sonidegib have demonstrated effectiveness for the treatment of basal cell carcinoma (BCC), their clinical use has been hampered by severe side effects, low selectivity against cancer stem cells, and the onset of mutation-driven drug resistance. Moreover, SMO antagonists are not effective in cancers where HH activation is due to mutations of pathway components downstream of SMO, or in the case of noncanonical, SMO-independent activation of the GLI transcription factors, the final mediators of HH signaling. Here, we review the current and rapidly expanding field of SMO small-molecule inhibitors in experimental and clinical settings, focusing on a class of acylguanidine derivatives. We also discuss various aspects of SMO, including mechanisms of resistance to SMO antagonists.
Keywords: GLI; acylguanidine derivative; cancer; drug-resistance; hedgehog; missense mutations; small molecule inhibitors; smoothened; targeted therapy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

