Human Respiratory Syncytial Virus NS 1 Targets TRIM25 to Suppress RIG-I Ubiquitination and Subsequent RIG-I-Mediated Antiviral Signaling
- PMID: 30558248
- PMCID: PMC6316657
- DOI: 10.3390/v10120716
Human Respiratory Syncytial Virus NS 1 Targets TRIM25 to Suppress RIG-I Ubiquitination and Subsequent RIG-I-Mediated Antiviral Signaling
Abstract
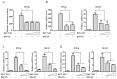
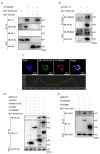
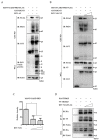
Respiratory syncytial virus (RSV) causes severe acute lower respiratory tract disease. Retinoic acid-inducible gene-I (RIG-I) serves as an innate immune sensor and triggers antiviral responses upon recognizing viral infections including RSV. Since tripartite motif-containing protein 25 (TRIM25)-mediated K63-polyubiquitination is crucial for RIG-I activation, several viruses target initial RIG-I activation through ubiquitination. RSV NS1 and NS2 have been shown to interfere with RIG-I-mediated antiviral signaling. In this study, we explored the possibility that NS1 suppresses RIG-I-mediated antiviral signaling by targeting TRIM25. Ubiquitination of ectopically expressed RIG-I-2Cards domain was decreased by RSV infection, indicating that RSV possesses ability to inhibit TRIM25-mediated RIG-I ubiquitination. Similarly, ectopic expression of NS1 sufficiently suppressed TRIM25-mediated RIG-I ubiquitination. Furthermore, interaction between NS1 and TRIM25 was detected by a co-immunoprecipitation assay. Further biochemical assays showed that the SPRY domain of TRIM25, which is responsible for interaction with RIG-I, interacted sufficiently with NS1. Suppression of RIG-I ubiquitination by NS1 resulted in decreased interaction between RIG-I and its downstream molecule, MAVS. The suppressive effect of NS1 on RIG-I signaling could be abrogated by overexpression of TRIM25. Collectively, this study suggests that RSV NS1 interacts with TRIM25 and interferes with RIG-I ubiquitination to suppress type-I interferon signaling.
Keywords: Interferon; Nonstructural Protein 1; RIG-I; Respiratory Syncytial Virus; TRIM25.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
A RIG-I-like receptor directs antiviral responses to a bunyavirus and is antagonized by virus-induced blockade of TRIM25-mediated ubiquitination.J Biol Chem. 2020 Jul 10;295(28):9691-9711. doi: 10.1074/jbc.RA120.013973. Epub 2020 May 29. J Biol Chem. 2020. PMID: 32471869 Free PMC article.
-
The Human Papillomavirus E6 Oncoprotein Targets USP15 and TRIM25 To Suppress RIG-I-Mediated Innate Immune Signaling.J Virol. 2018 Feb 26;92(6):e01737-17. doi: 10.1128/JVI.01737-17. Print 2018 Mar 15. J Virol. 2018. PMID: 29263274 Free PMC article.
-
Human metapneumovirus M2-2 protein inhibits RIG-I signaling by preventing TRIM25-mediated RIG-I ubiquitination.Front Immunol. 2022 Aug 15;13:970750. doi: 10.3389/fimmu.2022.970750. eCollection 2022. Front Immunol. 2022. PMID: 36045682 Free PMC article.
-
Respiratory syncytial virus nonstructural proteins 1 and 2: Exceptional disrupters of innate immune responses.PLoS Pathog. 2019 Oct 17;15(10):e1007984. doi: 10.1371/journal.ppat.1007984. eCollection 2019 Oct. PLoS Pathog. 2019. PMID: 31622448 Free PMC article. Review.
-
TRIM proteins: another class of viral victims.Sci Signal. 2010 Apr 20;3(118):jc2. doi: 10.1126/scisignal.3118jc2. Sci Signal. 2010. PMID: 20407122 Review.
Cited by
-
Safety and Effectiveness of Inhaling Different Dosage Recombinant Human Interferon α1B for Bronchiolitis in Children: a Systematic Review and Meta-Analysis.Evid Based Complement Alternat Med. 2022 Apr 27;2022:2229735. doi: 10.1155/2022/2229735. eCollection 2022. Evid Based Complement Alternat Med. 2022. PMID: 35529920 Free PMC article.
-
Molecular basis for human respiratory syncytial virus transcriptional regulator NS1 interactions with MED25.Nat Commun. 2025 Mar 25;16(1):2883. doi: 10.1038/s41467-025-58216-4. Nat Commun. 2025. PMID: 40128225 Free PMC article.
-
An Unexpected Encounter: Respiratory Syncytial Virus Nonstructural Protein 1 Interacts with Mediator Subunit MED25.J Virol. 2022 Oct 12;96(19):e0129722. doi: 10.1128/jvi.01297-22. Epub 2022 Sep 14. J Virol. 2022. PMID: 36102648 Free PMC article. Review.
-
Intracellular sensing of viral genomes and viral evasion.Exp Mol Med. 2019 Dec 11;51(12):1-13. doi: 10.1038/s12276-019-0299-y. Exp Mol Med. 2019. PMID: 31827068 Free PMC article. Review.
-
Mechanisms of Non-segmented Negative Sense RNA Viral Antagonism of Host RIG-I-Like Receptors.J Mol Biol. 2019 Oct 4;431(21):4281-4289. doi: 10.1016/j.jmb.2019.06.002. Epub 2019 Jun 14. J Mol Biol. 2019. PMID: 31202887 Free PMC article. Review.
References
-
- Nair H., Nokes D.J., Gessner B.D., Dherani M., Madhi S.A., Singleton R.J., O’Brien K.L., Roca A., Wright P.F., Bruce N., et al. Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: A systematic review and meta-analysis. Lancet. 2010;375:1545–1555. doi: 10.1016/S0140-6736(10)60206-1. - DOI - PMC - PubMed
-
- Liu P., Jamaluddin M., Li K., Garofalo R.P., Casola A., Brasier A.R. Retinoic acid-inducible gene I mediates early antiviral response and Toll-like receptor 3 expression in respiratory syncytial virus-infected airway epithelial cells. J. Virol. 2007;81:1401–1411. doi: 10.1128/JVI.01740-06. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

