Induced Resistance Mechanism of Novel Curcumin Analogs Bearing a Quinazoline Moiety to Plant Virus
- PMID: 30558295
- PMCID: PMC6321402
- DOI: 10.3390/ijms19124065
Induced Resistance Mechanism of Novel Curcumin Analogs Bearing a Quinazoline Moiety to Plant Virus
Abstract
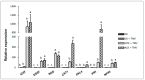
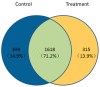
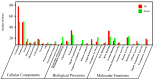
Plant immune activators can protect crops from plant virus pathogens by activating intrinsic immune mechanisms in plants and are widely used in agricultural production. In our previous work, we found that curcumin analogs exhibit excellent biological activity against plant viruses, especially protective activity. Inspired by these results, the active substructure of pentadienone and quinazoline were spliced to obtain curcumin analogs as potential exogenously induced resistant molecule. Bioassay results showed that compound A13 exhibited excellent protective activity for tobacco to against Tobacco mosaic virus (TMV) at 500 μg/mL, with a value of 70.4 ± 2.6% compared with control treatments, which was better than that of the plant immune activator chitosan oligosaccharide (49.0 ± 5.9%). The protective activity is due to compound A13 inducing tobacco resistance to TMV, which was related to defense-related enzymes, defense-related genes, and photosynthesis. This was confirmed by the up-regulated expression of proteins that mediate stress responses and oxidative phosphorylation.
Keywords: curcumin analogs; plant induced resistance; protective activity; quinazoline moiety; tobacco mosaic virus.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
Syntheses, antiviral activities and induced resistance mechanisms of novel quinazoline derivatives containing a dithioacetal moiety.Bioorg Chem. 2018 Oct;80:433-443. doi: 10.1016/j.bioorg.2018.06.026. Epub 2018 Jun 20. Bioorg Chem. 2018. PMID: 29986188
-
Novel trans-Ferulic Acid Derivatives Containing a Chalcone Moiety as Potential Activator for Plant Resistance Induction.J Agric Food Chem. 2017 Jun 7;65(22):4367-4377. doi: 10.1021/acs.jafc.7b00958. Epub 2017 Apr 25. J Agric Food Chem. 2017. PMID: 28368612
-
Alpha-momorcharin enhances Nicotiana benthamiana resistance to tobacco mosaic virus infection through modulation of reactive oxygen species.Mol Plant Pathol. 2020 Sep;21(9):1212-1226. doi: 10.1111/mpp.12974. Epub 2020 Jul 26. Mol Plant Pathol. 2020. PMID: 32713165 Free PMC article.
-
Spicing Up the N Gene: F. O. Holmes and Tobacco mosaic virus Resistance in Capsicum and Nicotiana Plants.Phytopathology. 2017 Feb;107(2):148-157. doi: 10.1094/PHYTO-07-16-0264-RVW. Epub 2016 Nov 30. Phytopathology. 2017. PMID: 27642796 Review.
-
Melatonin as a Possible Natural Anti-Viral Compound in Plant Biocontrol.Plants (Basel). 2023 Feb 9;12(4):781. doi: 10.3390/plants12040781. Plants (Basel). 2023. PMID: 36840129 Free PMC article. Review.
Cited by
-
Design, Synthesis, Antiviral Bioactivity, and Mechanism of the Ferulic Acid Ester-Containing Sulfonamide Moiety.ACS Omega. 2020 Jul 29;5(31):19721-19726. doi: 10.1021/acsomega.0c02421. eCollection 2020 Aug 11. ACS Omega. 2020. PMID: 32803067 Free PMC article.
-
Sulfur Induces Resistance against Canker Caused by Pseudomonas syringae pv. actinidae via Phenolic Components Increase and Morphological Structure Modification in the Kiwifruit Stems.Int J Mol Sci. 2021 Nov 10;22(22):12185. doi: 10.3390/ijms222212185. Int J Mol Sci. 2021. PMID: 34830066 Free PMC article.
-
Design, Synthesis, and Bioactivity Evaluation of Novel Isoxazole-Amide Derivatives Containing an Acylhydrazone Moiety as New Active Antiviral Agents.Molecules. 2019 Oct 19;24(20):3766. doi: 10.3390/molecules24203766. Molecules. 2019. PMID: 31635044 Free PMC article.
References
-
- Panattoni A., Luvisi A., Triolo E. Selective chemotherapy on Grapevine leafroll-associated virus-1 and -3. Phytoparasitica. 2011;39:503–508. doi: 10.1007/s12600-011-0185-1. - DOI
-
- Silva N.L., Zobiole N.N., Brentan da Silva D., Sartori A.L.B., Oliveira R.J., Pinto M.E.A., Leite dos Santos F.J., Maximo de Siqueira J. Chemical constituents and phytotoxic activity of leaves of Annona nutans. Quim Nova. 2015;38:640–644. doi: 10.5935/0100-4042.20150066. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

