Fingerprints of sp¹ Hybridized C in the Near-Edge X-ray Absorption Spectra of Surface-Grown Materials
- PMID: 30558338
- PMCID: PMC6315668
- DOI: 10.3390/ma11122556
Fingerprints of sp¹ Hybridized C in the Near-Edge X-ray Absorption Spectra of Surface-Grown Materials
Abstract
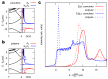
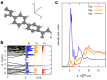
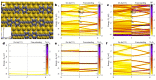
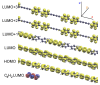
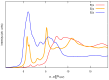
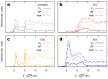
Carbon structures comprising sp 1 chains (e.g., polyynes or cumulenes) can be synthesized by exploiting on-surface chemistry and molecular self-assembly of organic precursors, opening to the use of the full experimental and theoretical surface-science toolbox for their characterization. In particular, polarized near-edge X-ray absorption fine structure (NEXAFS) can be used to determine molecular adsorption angles and is here also suggested as a probe to discriminate sp 1 /sp 2 character in the structures. We present an ab initio study of the polarized NEXAFS spectrum of model and real sp 1 /sp 2 materials. Calculations are performed within density functional theory with plane waves and pseudopotentials, and spectra are computed by core-excited C potentials. We evaluate the dichroism in the spectrum for ideal carbynes and highlight the main differences relative to typical sp 2 systems. We then consider a mixed polymer alternating sp 1 C 4 units with sp 2 biphenyl groups, recently synthesized on Au(111), as well as other linear structures and two-dimensional networks, pointing out a spectral line shape specifically due to the the presence of linear C chains. Our study suggests that the measurements of polarized NEXAFS spectra could be used to distinctly fingerprint the presence of sp 1 hybridization in surface-grown C structures.
Keywords: C 1s absorption; carbynes; density functional theory; near edge X-ray absorption spectroscopy; on-surface chemistry; self-assembly.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Matrix effects in the carbon 1s near edge x-ray absorption fine structure spectra of condensed alkanes.J Chem Phys. 2006 Jun 21;124(23):234704. doi: 10.1063/1.2206589. J Chem Phys. 2006. PMID: 16821938
-
First-principles simulation of X-ray spectra of graphdiyne and graphdiyne oxides at the carbon K-edge.Phys Chem Chem Phys. 2023 Dec 6;25(47):32421-32429. doi: 10.1039/d3cp03193d. Phys Chem Chem Phys. 2023. PMID: 37782052
-
Self-assembled carbon nanotubes on gold: polarization-modulated infrared reflection-absorption spectroscopy, high-resolution X-ray photoemission spectroscopy, and near-edge X-ray absorption fine structure spectroscopy study.Langmuir. 2008 Apr 1;24(7):3235-43. doi: 10.1021/la7030768. Epub 2008 Feb 19. Langmuir. 2008. PMID: 18281998
-
Quantitative Evaluation of the Carbon Hybridization State by Near Edge X-ray Absorption Fine Structure Spectroscopy.Anal Chem. 2016 Mar 1;88(5):2817-24. doi: 10.1021/acs.analchem.5b04525. Epub 2016 Feb 12. Anal Chem. 2016. PMID: 26814796
-
Rydberg-valence mixing in the carbon 1s near-edge X-ray absorption fine structure spectra of gaseous alkanes.J Phys Chem A. 2005 Mar 17;109(10):2151-9. doi: 10.1021/jp045370e. J Phys Chem A. 2005. PMID: 16838986
Cited by
-
Structural, Electronic, and Vibrational Properties of a Two-Dimensional Graphdiyne-like Carbon Nanonetwork Synthesized on Au(111): Implications for the Engineering of sp-sp2 Carbon Nanostructures.ACS Appl Nano Mater. 2020 Dec 24;3(12):12178-12187. doi: 10.1021/acsanm.0c02665. Epub 2020 Dec 4. ACS Appl Nano Mater. 2020. PMID: 33392466 Free PMC article.
References
-
- Castelli I.E., Salvestrini P., Manini N. Mechanical properties of carbynes investigated by ab-initio total-energy calculations. Phys. Rev. B. 2012;85:214110. doi: 10.1103/PhysRevB.85.214110. - DOI
-
- Delodovici F., Manini N., Wittman R., Choi D., Fahim M.A., Burchfield L. Protomene: A new carbon allotrope. Carbon. 2018;126:574. doi: 10.1016/j.carbon.2017.10.069. - DOI

