Effects of a New Bioceramic Material on Human Apical Papilla Cells
- PMID: 30558359
- PMCID: PMC6306901
- DOI: 10.3390/jfb9040074
Effects of a New Bioceramic Material on Human Apical Papilla Cells
Abstract
Background: The development of materials with bioregenerative properties is critically important for vital pulp therapies and regenerative endodontic procedures. The aim of this study was to evaluate the cytocompatibility and cytotoxicity of a new endodontic biomaterial, PulpGuard, in comparison with two other biomaterials widely used in endodontic procedures, ProRoot Mineral Trioxide Aggregate (MTA) and Biodentine.
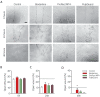
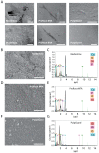
Methods: Apical papilla cells (APCs) were isolated from third molars with incomplete rhizogenesis from patients with orthodontic indication for dental extraction. Cultured APCs were incubated for 24, 48, or 72 h with different dilutions of eluates prepared from the three materials. Cellular viability, mobility, and proliferation were assessed in vitro using the Alamar Blue assay and a wound-healing test. The cells were also cultured in direct contact with the surface of each material. These were then analyzed via Scanning Electron Microscopy (SEM), and the surface chemical composition was determined by Energy-Dispersive Spectroscopy (EDS).
Results: Cells incubated in the presence of eluates extracted from ProRoot MTA and PulpGuard presented rates of viability comparable to those of control cells; in contrast, undiluted Biodentine eluates induced a significant reduction of cellular viability. The wound-healing assay revealed that eluates from ProRoot MTA and PulpGuard allowed for unhindered cellular migration and proliferation. Cellular adhesion was observed on the surface of all materials tested. Consistent with their disclosed composition, EDS analysis found high relative abundance of calcium in Biodentine and ProRoot MTA and high abundance of silicon in PulpGuard. Significant amounts of zinc and calcium were also present in PulpGuard discs. Concerning solubility, Biodentine and ProRoot MTA presented mild weight loss after eluate extraction, while PulpGuard discs showed significant water uptake.
Conclusions: PulpGuard displayed a good in vitro cytocompatibility profile and did not significantly affect the proliferation and migration rates of APCs. Cells cultured in the presence of PulpGuard eluates displayed a similar profile to those cultured with eluates from the widely used endodontic cement ProRoot MTA.
Keywords: PulpGuard; SCAPS; biocompatibility; calcium silicate cements; cytotoxicity; regenerative endodontics.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Gandolfi M.G., Spagnuolo G., Siboni F., Procino A., Rivieccio V., Pelliccioni G.A., Prati C., Rengo S. Calcium silicate/calcium phosphate biphasic cements for vital pulp therapy: Chemical-physical properties and human pulp cells response. Clin. Oral Investig. 2015;19:2075–2089. doi: 10.1007/s00784-015-1443-2. - DOI - PubMed
-
- Rodríguez-Lozano F.J., García-Bernal D., Oñate-Sánchez R.E., Ortolani-Seltenerich P.S., Forner L., Moraleda J.M. Evaluation of cytocompatibility of calcium silicate-based endodontic sealers and their effects on the biological responses of mesenchymal dental stem cells. Int. Endod. J. 2017;50:67–76. doi: 10.1111/iej.12596. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

