Removal Effect of Atrazine in Co-Solution with Bisphenol A or Humic Acid by Different Activated Carbons
- PMID: 30558368
- PMCID: PMC6316426
- DOI: 10.3390/ma11122558
Removal Effect of Atrazine in Co-Solution with Bisphenol A or Humic Acid by Different Activated Carbons
Abstract
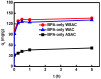
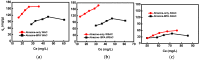
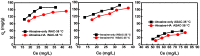
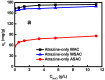
Activated carbons (ACs) based on apricot shells (AS), wood (W), and walnut shells (WS) were applied to adsorb atrazine in co-solutions. To study the effect of Bisphenol A (BPA) on the adsorption behavior of atrazine, the adsorption performance of ACs for BPA in single solution was studied. The results demonstrated that the adsorption kinetics of BPA fitted the pseudo-second-order model, the adsorption isotherms of BPA followed the Langmuir model. Meanwhile, the adsorption kinetics of atrazine fitted the pseudo-second-order kinetics model and the isotherm was consistent with the Freundlich model both in single solution and co-solution. In addition, competitive adsorption was observed when atrazine coexisted with BPA or humic acid. For the adsorption capacity, the adsorption amount of ASAC, WAC, and WSAC for atrazine obviously decreased by 18.0%, 30.0%, and 30.3% in the presence of BPA, respectively, which was due to the π-π interactions, hydrophobic interactions, and H-bonds, resulting in the competitive adsorption between atrazine and BPA. This study contributes to the further understanding of the adsorption behavior for atrazine in co-solution.
Keywords: NaCl; activated carbon; adsorption; atrazine; bisphenol A; co-solution; humic acids.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

