A Gemini Cationic Lipid with Histidine Residues as a Novel Lipid-Based Gene Nanocarrier: A Biophysical and Biochemical Study
- PMID: 30558369
- PMCID: PMC6316511
- DOI: 10.3390/nano8121061
A Gemini Cationic Lipid with Histidine Residues as a Novel Lipid-Based Gene Nanocarrier: A Biophysical and Biochemical Study
Abstract
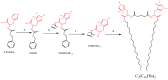
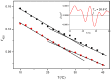
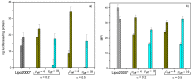
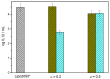
This work reports the synthesis of a novel gemini cationic lipid that incorporates two histidine-type head groups (C₃(C16His)₂). Mixed with a helper lipid 1,2-dioleoyl-sn-glycero-3-phosphatidyl ethanol amine (DOPE), it was used to transfect three different types of plasmid DNA: one encoding the green fluorescence protein (pEGFP-C3), one encoding a luciferase (pCMV-Luc), and a therapeutic anti-tumoral agent encoding interleukin-12 (pCMV-IL12). Complementary biophysical experiments (zeta potential, gel electrophoresis, small-angle X-ray scattering (SAXS), and fluorescence anisotropy) and biological studies (FACS, luminometry, and cytotoxicity) of these C₃(C16His)₂/DOPE-pDNA lipoplexes provided vast insight into their outcomes as gene carriers. They were found to efficiently compact and protect pDNA against DNase I degradation by forming nanoaggregates of 120⁻290 nm in size, which were further characterized as very fluidic lamellar structures based in a sandwich-type phase, with alternating layers of mixed lipids and an aqueous monolayer where the pDNA and counterions are located. The optimum formulations of these nanoaggregates were able to transfect the pDNAs into COS-7 and HeLa cells with high cell viability, comparable or superior to that of the standard Lipo2000*. The vast amount of information collected from the in vitro studies points to this histidine-based lipid nanocarrier as a potentially interesting candidate for future in vivo studies investigating specific gene therapies.
Keywords: biophysical characterization; cell viability; gemini cationic lipid with histidine residues; gene delivery; lipid-based gene nanocarrier; plasmid DNAs; transfection.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
Transfection of plasmid DNA by nanocarriers containing a gemini cationic lipid with an aromatic spacer or its monomeric counterpart.Colloids Surf B Biointerfaces. 2018 Jan 1;161:519-527. doi: 10.1016/j.colsurfb.2017.11.024. Epub 2017 Nov 8. Colloids Surf B Biointerfaces. 2018. PMID: 29128838
-
Multidisciplinary Approach to the Transfection of Plasmid DNA by a Nonviral Nanocarrier Based on a Gemini-Bolaamphiphilic Hybrid Lipid.ACS Omega. 2018 Jan 31;3(1):208-217. doi: 10.1021/acsomega.7b01657. Epub 2018 Jan 8. ACS Omega. 2018. PMID: 30023772 Free PMC article.
-
How does the spacer length of cationic gemini lipids influence the lipoplex formation with plasmid DNA? Physicochemical and biochemical characterizations and their relevance in gene therapy.Biomacromolecules. 2012 Dec 10;13(12):3926-37. doi: 10.1021/bm301066w. Epub 2012 Nov 16. Biomacromolecules. 2012. PMID: 23130552
-
Ca(2+)-mediated anionic lipid-plasmid DNA lipoplexes. Electrochemical, structural, and biochemical studies.Langmuir. 2014 Oct 7;30(39):11704-13. doi: 10.1021/la502823z. Epub 2014 Sep 25. Langmuir. 2014. PMID: 25211646
-
Recent progress in gene therapy to deliver nucleic acids with multivalent cationic vectors.Adv Colloid Interface Sci. 2016 Jul;233:161-175. doi: 10.1016/j.cis.2015.07.003. Epub 2015 Jul 26. Adv Colloid Interface Sci. 2016. PMID: 26265376 Review.
Cited by
-
Cationic Serine-Based Gemini Surfactant:Monoolein Aggregates as Viable and Efficacious Agents for DNA Complexation and Compaction: A Cytotoxicity and Physicochemical Assessment.J Funct Biomater. 2024 Aug 13;15(8):224. doi: 10.3390/jfb15080224. J Funct Biomater. 2024. PMID: 39194661 Free PMC article.
-
Protein Expression Knockdown in Cancer Cells Induced by a Gemini Cationic Lipid Nanovector with Histidine-Based Polar Heads.Pharmaceutics. 2020 Aug 21;12(9):791. doi: 10.3390/pharmaceutics12090791. Pharmaceutics. 2020. PMID: 32825658 Free PMC article.
-
The Process of Binding and Releasing of Genetic Material from Lipoplexes Based on Trimeric Surfactants and Phospholipids.Int J Mol Sci. 2021 Jul 20;22(14):7744. doi: 10.3390/ijms22147744. Int J Mol Sci. 2021. PMID: 34299360 Free PMC article.
-
Gemini Cationic Lipid-Type Nanovectors Suitable for the Transfection of Therapeutic Plasmid DNA Encoding for Pro-Inflammatory Cytokine Interleukin-12.Pharmaceutics. 2021 May 15;13(5):729. doi: 10.3390/pharmaceutics13050729. Pharmaceutics. 2021. PMID: 34063469 Free PMC article.
-
Ammonium Gemini Surfactants Form Complexes with Model Oligomers of siRNA and dsDNA.Int J Mol Sci. 2019 Nov 7;20(22):5546. doi: 10.3390/ijms20225546. Int J Mol Sci. 2019. PMID: 31703275 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

