Expression of FGD4 positively correlates with the aggressive phenotype of prostate cancer
- PMID: 30558664
- PMCID: PMC6296060
- DOI: 10.1186/s12885-018-5096-9
Expression of FGD4 positively correlates with the aggressive phenotype of prostate cancer
Abstract
Background: FGD4 (Frabin) is an F-actin binding protein with GTP/GDP exchange activity specific for CDC42. It is involved in reorganization of the actin cytoskeleton, which requires both actin binding and CDC42 activating function of FGD4. Expression of FGD4 is altered in patients with heterogeneous hereditary motor and sensory neuropathies as a result of demyelination of peripheral nerves.
Methods: In this study, we examined the expression of FGD4 in prostate cancer specimens using immunohistochemistry and studied the function of FGD4 in maintaining cell phenotype, behavior and drug sensitivity using overexpression and siRNA-based silencing approaches. We used Mann-Whitney test for comparative analysis of FGD4 expression.
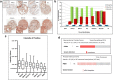
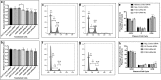
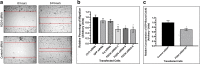
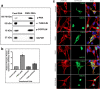
Results: Our results show that the expression of FGD4 is upregulated in cancerous prostates compared to the luminal cells in benign prostatic hyperplasia, although the basal cells showed high staining intensities. We noted a gradual increase in the staining intensity of FGD4 with increasing aggressiveness of the disease. Inhibition of expression of FGD4 using siRNAs showed reduced proliferation and cell cycle arrest in G2/M phase of androgen dependent LNCaP-104S and androgen refractory PC-3 cells. Inhibition of FGD4 also resulted in reduced cell migration and CDC42 activities in PC-3 cells whereas, ectopic expression of FGD4 induced cell migration, altered expression of mesenchymal and epithelial markers and activation of CDC42/PAK signaling pathway. Reduced expression of FGD4 improved sensitivity of LNCaP-104S cells to the anti-androgen drug Casodex and PC-3 cells to the microtubule stabilizing drug docetaxel.
Conclusions: Our data demonstrate a tumor promoting and a cell migratory function of FGD4 in prostate cancer cells and that inhibition of FGD4 expression enhances the response for both androgen-dependent and independent prostate cancer cells towards currently used prostate cancer drugs.
Keywords: Cell cycle; Cell migration; Drug sensitivity; Prostate cancer; Rho GEF.
Conflict of interest statement
Ethics approval and consent to participate
The prostate TMA construction was approved by the Moffitt scientific review committee (Reference # MCC13579) and by the USF’s institutional review board (IRB # USF 101642).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures



References
-
- Obaishi H, et al. Frabin, a novel FGD1-related actin filament-binding protein capable of changing cell shape and activating c-Jun N-terminal kinase. J Biol Chem. 1998;273(30):18697–18700. - PubMed
-
- Takai Y, Sasaki T, Matozaki T. Small GTP-binding proteins. Physiol Rev. 2001;81(1):153–208. - PubMed
-
- Hall A. Rho GTPases and the control of cell behaviour. Biochem Soc Trans. 2005;33(5):891–895. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

