Canine and human sinoatrial node: differences and similarities in the structure, function, molecular profiles, and arrhythmia
- PMID: 30559056
- PMCID: PMC6436970
- DOI: 10.1016/j.jvc.2018.10.004
Canine and human sinoatrial node: differences and similarities in the structure, function, molecular profiles, and arrhythmia
Abstract
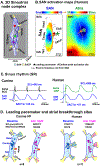
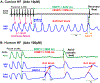
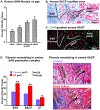
The sinoatrial node (SAN) is the primary pacemaker in canine and human hearts. The SAN in both species has a unique three-dimensional heterogeneous structure characterized by small pacemaker myocytes enmeshed within fibrotic strands, which partially insulate the cells from aberrant atrial activation. The SAN pacemaker tissue expresses a unique signature of proteins and receptors that mediate SAN automaticity, ion channel currents, and cell-to-cell communication, which are predominantly similar in both species. Recent intramural optical mapping, integrated with structural and molecular studies, has revealed the existence of up to five specialized SAN conduction pathways that preferentially conduct electrical activation to atrial tissues. The intrinsic heart rate, intranodal leading pacemaker shifts, and changes in conduction in response to physiological and pathophysiological stimuli are similar. Structural and/or functional impairments due to cardiac diseases including heart failure cause SAN dysfunctions (SNDs) in both species. These dysfunctions are usually manifested as severe bradycardia, tachy-brady arrhythmias, and conduction abnormalities including exit block and SAN reentry, which could lead to atrial tachycardia and fibrillation, cardiac arrest, and heart failure. Pharmaceutical drugs and implantable pacemakers are only partially successful in managing SNDs, emphasizing a critical need to develop targeted mechanism-based therapies to treat SNDs. Because several structural and functional characteristics are similar between the canine and human SAN, research in these species may be mutually beneficial for developing novel treatment approaches. This review describes structural, functional, and molecular similarities and differences between the canine and human SAN, with special emphasis on arrhythmias and unique causal mechanisms of SND in diseased hearts.
Keywords: Atrial fibrillation; Fibrosis; Pacemakers; Sick sinus syndrome; Sinoatrial node dysfunction.
Copyright © 2018 Elsevier B.V. All rights reserved.
Conflict of interest statement
Conflicts of Interest Statement
The authors do not have any conflicts to disclose.
Figures



Similar articles
-
Upregulation of adenosine A1 receptors facilitates sinoatrial node dysfunction in chronic canine heart failure by exacerbating nodal conduction abnormalities revealed by novel dual-sided intramural optical mapping.Circulation. 2014 Jul 22;130(4):315-24. doi: 10.1161/CIRCULATIONAHA.113.007086. Epub 2014 May 16. Circulation. 2014. PMID: 24838362 Free PMC article.
-
Sinoatrial node reentry in a canine chronic left ventricular infarct model: role of intranodal fibrosis and heterogeneity of refractoriness.Circ Arrhythm Electrophysiol. 2013 Oct;6(5):984-94. doi: 10.1161/CIRCEP.113.000404. Epub 2013 Aug 19. Circ Arrhythm Electrophysiol. 2013. PMID: 23960214 Free PMC article.
-
Conduction barriers and pathways of the sinoatrial pacemaker complex: their role in normal rhythm and atrial arrhythmias.Am J Physiol Heart Circ Physiol. 2012 May 1;302(9):H1773-83. doi: 10.1152/ajpheart.00892.2011. Epub 2012 Jan 20. Am J Physiol Heart Circ Physiol. 2012. PMID: 22268110 Review.
-
Tachy-brady arrhythmias: the critical role of adenosine-induced sinoatrial conduction block in post-tachycardia pauses.Heart Rhythm. 2013 Jan;10(1):110-8. doi: 10.1016/j.hrthm.2012.09.012. Epub 2012 Sep 14. Heart Rhythm. 2013. PMID: 22985657 Free PMC article.
-
Three-dimensional functional anatomy of the human sinoatrial node for epicardial and endocardial mapping and ablation.Heart Rhythm. 2023 Jan;20(1):122-133. doi: 10.1016/j.hrthm.2022.08.039. Epub 2022 Sep 14. Heart Rhythm. 2023. PMID: 36113768 Free PMC article. Review.
Cited by
-
Local tissue mechanics control cardiac pacemaker cell embryonic patterning.Life Sci Alliance. 2023 Mar 27;6(6):e202201799. doi: 10.26508/lsa.202201799. Print 2023 Jun. Life Sci Alliance. 2023. PMID: 36973005 Free PMC article.
-
Novel Insights into the Sinoatrial Node in Single-Cell RNA Sequencing: From Developmental Biology to Physiological Function.J Cardiovasc Dev Dis. 2022 Nov 18;9(11):402. doi: 10.3390/jcdd9110402. J Cardiovasc Dev Dis. 2022. PMID: 36421937 Free PMC article. Review.
-
Altered microRNA and mRNA profiles during heart failure in the human sinoatrial node.Sci Rep. 2021 Sep 29;11(1):19328. doi: 10.1038/s41598-021-98580-x. Sci Rep. 2021. PMID: 34588502 Free PMC article.
-
Mitochondrial dysfunction is a key link involved in the pathogenesis of sick sinus syndrome: a review.Front Cardiovasc Med. 2024 Oct 29;11:1488207. doi: 10.3389/fcvm.2024.1488207. eCollection 2024. Front Cardiovasc Med. 2024. PMID: 39534498 Free PMC article. Review.
-
Mechanistic insight into the functional role of human sinoatrial node conduction pathways and pacemaker compartments heterogeneity: A computer model analysis.PLoS Comput Biol. 2023 Dec 18;19(12):e1011708. doi: 10.1371/journal.pcbi.1011708. eCollection 2023 Dec. PLoS Comput Biol. 2023. PMID: 38109436 Free PMC article.
References
-
- Lewis T, Oppenheimer A, Oppenheimer BS. The site of origin of the mammalian heart beat: the pacemaker in the dog. Heart 1910;II:147–69.
-
- Schuessler RB, Boineau JP, Bromberg BI. Origin of the Sinus Impulse. J Cardiovasc Electrophysiol 1996;7:263–74. - PubMed
-
- James TN, Sherf L, Fine G, Morales AR. Comparative ultrastructure of the sinus node in man and dog. Circulation 1966;34:139–63. - PubMed
-
- Luu M, Stevenson WG, Stevenson LW, Baron K, Walden J. Diverse mechanisms of unexpected cardiac arrest in advanced heart failure. Circulation 1989;80:1675–80. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

