Changing Molecular Markers of Antimalarial Drug Sensitivity across Uganda
- PMID: 30559133
- PMCID: PMC6395896
- DOI: 10.1128/AAC.01818-18
Changing Molecular Markers of Antimalarial Drug Sensitivity across Uganda
Abstract
The potential spread of antimalarial drug resistance to Africa, in particular for artemisinins and key partner drugs, is a major concern. We surveyed Plasmodium falciparum genetic markers associated with drug sensitivity on 3 occasions at ∼6-month intervals in 2016 and 2017 at 10 sites representing a range of epidemiological settings in Uganda. For putative drug transporters, we found continued evolution toward wild-type sequences associated with increased sensitivity to chloroquine. For pfcrt K76T, by 2017 the prevalence of the wild type was >60% at all sites and >90% at 6 sites. For the pfmdr1 N86Y and D1246Y alleles, wild type prevalence ranged from 80 to 100%. We found low prevalence of K13 propeller domain mutations, which are associated with artemisinin resistance in Asia, but one mutation previously identified in northern Uganda, 675V, was seen in 2.0% of samples, including 5.5% of those from the 3 northernmost sites. Amplification of the pfmdr1 and plasmepsin2 genes, associated elsewhere with decreased sensitivity to lumefantrine and piperaquine, respectively, was seen in <1% of samples. For the antifolate targets pfdhfr and pfdhps, 5 mutations previously associated with resistance were very common, and the pfdhfr 164L and pfdhps 581G mutations associated with higher-level resistance were seen at multiple sites, although prevalence did not clearly increase over time. Overall, changes were consistent with the selective pressure of the national treatment regimen, artemether-lumefantrine, with increased sensitivity to chloroquine, and with poor efficacy of antifolates. Strong evidence for resistance to artemisinins was not seen. Continued surveillance of markers that predict antimalarial drug sensitivity is warranted.
Keywords: Uganda; antimalarial drug sensitivity; molecular markers.
Copyright © 2019 American Society for Microbiology.
Figures
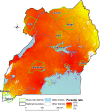
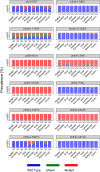
Comment in
-
Prevalence of Antimalarial Resistance Mediators.J Infect Dis. 2021 Mar 29;223(6):927-929. doi: 10.1093/infdis/jiaa688. J Infect Dis. 2021. PMID: 33146721 No abstract available.
References
-
- Yeka A, Gasasira A, Mpimbaza A, Achan J, Nankabirwa J, Nsobya S, Staedke SG, Donnelly MJ, Wabwire-Mangen F, Talisuna A, Dorsey G, Kamya MR, Rosenthal PJ. 2012. Malaria in Uganda: challenges to control on the long road to elimination: I. Epidemiology and current control efforts. Acta Trop 121:184–195. doi:10.1016/j.actatropica.2011.03.004. - DOI - PMC - PubMed
-
- Nankabirwa JI, Wandera B, Amuge P, Kiwanuka N, Dorsey G, Rosenthal PJ, Brooker SJ, Staedke SG, Kamya MR. 2014. Impact of intermittent preventive treatment with dihydroartemisinin-piperaquine on malaria in Ugandan schoolchildren: a randomized, placebo-controlled trial. Clin Infect Dis 58:1404–1412. doi:10.1093/cid/ciu150. - DOI - PMC - PubMed
-
- Cissé B, Ba EH, Sokhna C, NDiaye JL, Gomis JF, Dial Y, Pitt C, NDiaye M, Cairns M, Faye E, NDiaye M, Lo A, Tine R, Faye S, Faye B, Sy O, Konate L, Kouevijdin E, Flach C, Faye O, Trape J-F, Sutherland C, Fall FB, Thior PM, Faye OK, Greenwood B, Gaye O, Milligan P. 2016. Effectiveness of seasonal malaria chemoprevention in children under ten years of age in Senegal: a stepped-wedge cluster-randomised trial. PLoS Med 13:e1002175. doi:10.1371/journal.pmed.1002175. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

