Impact of existing vaccines in reducing antibiotic resistance: Primary and secondary effects
- PMID: 30559195
- PMCID: PMC6304973
- DOI: 10.1073/pnas.1721095115
Impact of existing vaccines in reducing antibiotic resistance: Primary and secondary effects
Abstract
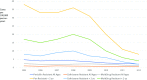
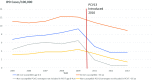
Vaccines impact antibiotic-resistant infections in two ways: through a direct reduction in the organisms and strains carrying resistant genes that are specifically targeted by the vaccine and also via a secondary effect through a reduction in febrile illnesses that often lead to the use of antibiotics. We review here the impact of pneumococcal conjugate vaccines (PCVs) on the prevalence of antibiotic-resistant disease and antibiotic usage as an example of the direct effect of vaccines on antibiotic resistance and the impact of influenza vaccination on antibiotic usage as an example of a secondary effect. A prelicensure study of a PCV in Africa demonstrated 67% fewer penicillin-resistant invasive disease episodes in the PCV group compared with controls. Similar studies in the United States and Europe demonstrated reductions in antibiotic use consistent with the vaccines' impact on the risk of otitis media infections in children. Postlicensure reductions in the circulation of antibiotic-resistant strains targeted by the vaccines have been dramatic, with virtual elimination of these strains in children following vaccine introduction. In terms of a secondary effect, following influenza vaccination reductions of 13-50% have been observed in the use of antibiotics by individuals receiving influenza vaccine compared with controls. With the demonstrated effectiveness of vaccination programs in impacting the risk of antibiotic-resistant infections and the increasing threat to public health that these infections represent, more attention needs to be given to development and utilization of vaccines to address antibiotic resistance.
Keywords: antibiotic resistance; influenza vaccine; pneumococcal vaccine; vaccines.
Conflict of interest statement
Conflict of interest statement: S.B. is a consultant for GSK, Merck, Sutrovax, and WHO.
Figures
References
-
- Magiorakos A-P, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012;18:268–281. - PubMed
-
- Pletz MW, Maus U, Krug N, Welte T, Lode H. Pneumococcal vaccines: Mechanism of action, impact on epidemiology and adaption of the species. Int J Antimicrob Agents. 2008;32:199–206. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical