Human induced pluripotent stem cell-derived MGE cell grafting after status epilepticus attenuates chronic epilepsy and comorbidities via synaptic integration
- PMID: 30559206
- PMCID: PMC6320542
- DOI: 10.1073/pnas.1814185115
Human induced pluripotent stem cell-derived MGE cell grafting after status epilepticus attenuates chronic epilepsy and comorbidities via synaptic integration
Abstract
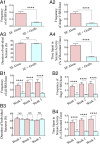
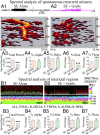
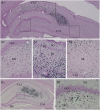
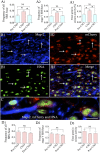
Medial ganglionic eminence (MGE)-like interneuron precursors derived from human induced pluripotent stem cells (hiPSCs) are ideal for developing patient-specific cell therapy in temporal lobe epilepsy (TLE). However, their efficacy for alleviating spontaneous recurrent seizures (SRS) or cognitive, memory, and mood impairments has never been tested in models of TLE. Through comprehensive video- electroencephalographic recordings and a battery of behavioral tests in a rat model, we demonstrate that grafting of hiPSC-derived MGE-like interneuron precursors into the hippocampus after status epilepticus (SE) greatly restrained SRS and alleviated cognitive, memory, and mood dysfunction in the chronic phase of TLE. Graft-derived cells survived well, extensively migrated into different subfields of the hippocampus, and differentiated into distinct subclasses of inhibitory interneurons expressing various calcium-binding proteins and neuropeptides. Moreover, grafting of hiPSC-MGE cells after SE mediated several neuroprotective and antiepileptogenic effects in the host hippocampus, as evidenced by reductions in host interneuron loss, abnormal neurogenesis, and aberrant mossy fiber sprouting in the dentate gyrus (DG). Furthermore, axons from graft-derived interneurons made synapses on the dendrites of host excitatory neurons in the DG and the CA1 subfield of the hippocampus, implying an excellent graft-host synaptic integration. Remarkably, seizure-suppressing effects of grafts were significantly reduced when the activity of graft-derived interneurons was silenced by a designer drug while using donor hiPSC-MGE cells expressing designer receptors exclusively activated by designer drugs (DREADDs). These results implied the direct involvement of graft-derived interneurons in seizure control likely through enhanced inhibitory synaptic transmission. Collectively, the results support a patient-specific MGE cell grafting approach for treating TLE.
Keywords: EEG recordings; GABA-ergic progenitors; cognition and mood; medial ganglionic eminence; temporal lobe epilepsy.
Copyright © 2019 the Author(s). Published by PNAS.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Neural Stem Cell or Human Induced Pluripotent Stem Cell-Derived GABA-ergic Progenitor Cell Grafting in an Animal Model of Chronic Temporal Lobe Epilepsy.Curr Protoc Stem Cell Biol. 2016 Aug 17;38:2D.7.1-2D.7.47. doi: 10.1002/cpsc.9. Curr Protoc Stem Cell Biol. 2016. PMID: 27532817 Free PMC article.
-
Medial ganglionic eminence-derived neural stem cell grafts ease spontaneous seizures and restore GDNF expression in a rat model of chronic temporal lobe epilepsy.Stem Cells. 2010 Jul;28(7):1153-64. doi: 10.1002/stem.446. Stem Cells. 2010. PMID: 20506409 Free PMC article.
-
Grafting of striatal precursor cells into hippocampus shortly after status epilepticus restrains chronic temporal lobe epilepsy.Exp Neurol. 2008 Aug;212(2):468-81. doi: 10.1016/j.expneurol.2008.04.040. Epub 2008 May 15. Exp Neurol. 2008. PMID: 18579133 Free PMC article.
-
GABA-ergic cell therapy for epilepsy: Advances, limitations and challenges.Neurosci Biobehav Rev. 2016 Mar;62:35-47. doi: 10.1016/j.neubiorev.2015.12.014. Epub 2015 Dec 31. Neurosci Biobehav Rev. 2016. PMID: 26748379 Free PMC article. Review.
-
Hippocampal injury-induced cognitive and mood dysfunction, altered neurogenesis, and epilepsy: can early neural stem cell grafting intervention provide protection?Epilepsy Behav. 2014 Sep;38:117-24. doi: 10.1016/j.yebeh.2013.12.001. Epub 2014 Jan 13. Epilepsy Behav. 2014. PMID: 24433836 Free PMC article. Review.
Cited by
-
FDA-approved cannabidiol [Epidiolex®] alleviates Gulf War Illness-linked cognitive and mood dysfunction, hyperalgesia, neuroinflammatory signaling, and declined neurogenesis.Mil Med Res. 2024 Aug 22;11(1):61. doi: 10.1186/s40779-024-00563-2. Mil Med Res. 2024. PMID: 39169440 Free PMC article.
-
Human neural stem cell transplants to address multiple pathologies associated with traumatic brain injury.Neural Regen Res. 2019 Oct;14(10):1699-1700. doi: 10.4103/1673-5374.255620. Neural Regen Res. 2019. PMID: 31169178 Free PMC article. No abstract available.
-
Stem Cell Therapy in Treating Epilepsy.Front Neurosci. 2022 Jun 27;16:934507. doi: 10.3389/fnins.2022.934507. eCollection 2022. Front Neurosci. 2022. PMID: 35833086 Free PMC article. Review.
-
Human Stem Cell-Derived GABAergic Interneurons Establish Efferent Synapses onto Host Neurons in Rat Epileptic Hippocampus and Inhibit Spontaneous Recurrent Seizures.Int J Mol Sci. 2021 Dec 8;22(24):13243. doi: 10.3390/ijms222413243. Int J Mol Sci. 2021. PMID: 34948040 Free PMC article.
-
Interneuron odyssey: molecular mechanisms of tangential migration.Front Neural Circuits. 2023 Sep 14;17:1256455. doi: 10.3389/fncir.2023.1256455. eCollection 2023. Front Neural Circuits. 2023. PMID: 37779671 Free PMC article. Review.
References
-
- Dudek FE, Hellier JL, Williams PA, Ferraro DJ, Staley KJ. The course of cellular alterations associated with the development of spontaneous seizures after status epilepticus. Prog Brain Res. 2002;135:53–65. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

