Structural determinants driving homoserine lactone ligand selection in the Pseudomonas aeruginosa LasR quorum-sensing receptor
- PMID: 30559209
- PMCID: PMC6320529
- DOI: 10.1073/pnas.1817239116
Structural determinants driving homoserine lactone ligand selection in the Pseudomonas aeruginosa LasR quorum-sensing receptor
Abstract
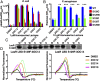
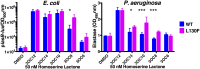
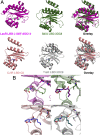
Quorum sensing is a cell-cell communication process that bacteria use to orchestrate group behaviors. Quorum sensing is mediated by signal molecules called autoinducers. Autoinducers are often structurally similar, raising questions concerning how bacteria distinguish among them. Here, we use the Pseudomonas aeruginosa LasR quorum-sensing receptor to explore signal discrimination. The cognate autoinducer, 3OC12 homoserine lactone (3OC12HSL), is a more potent activator of LasR than other homoserine lactones. However, other homoserine lactones can elicit LasR-dependent quorum-sensing responses, showing that LasR displays ligand promiscuity. We identify mutants that alter which homoserine lactones LasR detects. Substitution at residue S129 decreases the LasR response to 3OC12HSL, while enhancing discrimination against noncognate autoinducers. Conversely, the LasR L130F mutation increases the potency of 3OC12HSL and other homoserine lactones. We solve crystal structures of LasR ligand-binding domains complexed with noncognate autoinducers. Comparison with existing structures reveals that ligand selectivity/sensitivity is mediated by a flexible loop near the ligand-binding site. We show that LasR variants with modified ligand preferences exhibit altered quorum-sensing responses to autoinducers in vivo. We suggest that possessing some ligand promiscuity endows LasR with the ability to optimally regulate quorum-sensing traits.
Keywords: LasR; Pseudomonas aeruginosa; crystal structure; homoserine lactone; quorum sensing.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





Similar articles
-
An Autoinducer Analogue Reveals an Alternative Mode of Ligand Binding for the LasR Quorum-Sensing Receptor.ACS Chem Biol. 2019 Mar 15;14(3):378-389. doi: 10.1021/acschembio.8b00971. Epub 2019 Mar 4. ACS Chem Biol. 2019. PMID: 30763066 Free PMC article.
-
Molecular insights into quorum sensing in the human pathogen Pseudomonas aeruginosa from the structure of the virulence regulator LasR bound to its autoinducer.J Biol Chem. 2007 May 4;282(18):13592-600. doi: 10.1074/jbc.M700556200. Epub 2007 Mar 15. J Biol Chem. 2007. PMID: 17363368
-
Pseudomonas aeruginosa autoinducer enters and functions in mammalian cells.J Bacteriol. 2004 Apr;186(8):2281-7. doi: 10.1128/JB.186.8.2281-2287.2004. J Bacteriol. 2004. PMID: 15060029 Free PMC article.
-
New insights into the regulatory mechanisms of the LuxR family of quorum sensing regulators.Anal Bioanal Chem. 2007 Jan;387(2):381-90. doi: 10.1007/s00216-006-0702-0. Epub 2006 Sep 5. Anal Bioanal Chem. 2007. PMID: 16953322 Review.
-
Impact of quorum sensing on fitness of Pseudomonas aeruginosa.Int J Med Microbiol. 2006 Apr;296(2-3):93-102. doi: 10.1016/j.ijmm.2006.01.043. Epub 2006 Feb 28. Int J Med Microbiol. 2006. PMID: 16503417 Review.
Cited by
-
Natural and synthetic inhibitors of a phage-encoded quorum-sensing receptor affect phage-host dynamics in mixed bacterial communities.Proc Natl Acad Sci U S A. 2022 Dec 6;119(49):e2217813119. doi: 10.1073/pnas.2217813119. Epub 2022 Nov 29. Proc Natl Acad Sci U S A. 2022. PMID: 36445970 Free PMC article.
-
Computational and Biological Evaluation of β-Adrenoreceptor Blockers as Promising Bacterial Anti-Virulence Agents.Pharmaceuticals (Basel). 2022 Jan 18;15(2):110. doi: 10.3390/ph15020110. Pharmaceuticals (Basel). 2022. PMID: 35215223 Free PMC article.
-
Metabolic basis for the evolution of a common pathogenic Pseudomonas aeruginosa variant.Elife. 2022 May 3;11:e76555. doi: 10.7554/eLife.76555. Elife. 2022. PMID: 35502894 Free PMC article.
-
Molecular Mechanisms and Applications of N-Acyl Homoserine Lactone-Mediated Quorum Sensing in Bacteria.Molecules. 2022 Nov 4;27(21):7584. doi: 10.3390/molecules27217584. Molecules. 2022. PMID: 36364411 Free PMC article. Review.
-
The Antimicrobial Peptide Human Beta-Defensin 2 Inhibits Biofilm Production of Pseudomonas aeruginosa Without Compromising Metabolic Activity.Front Immunol. 2020 May 8;11:805. doi: 10.3389/fimmu.2020.00805. eCollection 2020. Front Immunol. 2020. PMID: 32457749 Free PMC article.
References
-
- Latifi A, et al. Multiple homologues of LuxR and LuxI control expression of virulence determinants and secondary metabolites through quorum sensing in Pseudomonas aeruginosa PAO1. Mol Microbiol. 1995;17:333–343. - PubMed
-
- Engebrecht J, Nealson K, Silverman M. Bacterial bioluminescence: Isolation and genetic analysis of functions from Vibrio fischeri. Cell. 1983;32:773–781. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources

