Mosquito Bite-Induced Controlled Human Malaria Infection with Plasmodium vivax or P. falciparum Generates Immune Responses to Homologous and Heterologous Preerythrocytic and Erythrocytic Antigens
- PMID: 30559218
- PMCID: PMC6386538
- DOI: 10.1128/IAI.00541-18
Mosquito Bite-Induced Controlled Human Malaria Infection with Plasmodium vivax or P. falciparum Generates Immune Responses to Homologous and Heterologous Preerythrocytic and Erythrocytic Antigens
Abstract
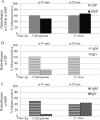
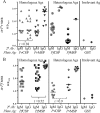
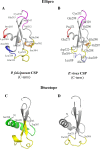
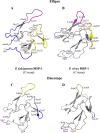
Seroepidemiological studies on the prevalence of antibodies to malaria antigens are primarily conducted on individuals from regions of endemicity. It is therefore difficult to accurately correlate the antibody responses to the timing and number of prior malaria infections. This study was undertaken to assess the evolution of antibodies to the dominant surface antigens of Plasmodium vivax and P. falciparum following controlled human malaria infection (CHMI) in malaria-naive individuals. Serum samples from malaria-naive adults, collected before and after CHMI with either P. vivax (n = 18) or P. falciparum (n = 18), were tested for the presence of antibodies to the circumsporozoite protein (CSP) and the 42-kDa fragment of merozoite surface protein 1 (MSP-142) of P. vivax and P. falciparum using an enzyme-linked immunosorbent assay (ELISA). Approximately 1 month following CHMI with either P. vivax or P. falciparum, >60% of subjects seroconverted to homologous CSP and MSP-1. More than 50% of the subjects demonstrated reactivity to heterologous CSP and MSP-142, and a similar proportion of subjects remained seropositive to homologous MSP-142 >5 months after CHMI. Computational analysis provides insight into the presence of cross-reactive responses. The presence of long-lived and heterologous reactivity and its functional significance, if any, need to be taken into account while evaluating malaria exposure in field settings.
Keywords: CHMI; CSP; MSP-1; Plasmodium falciparum; Plasmodium vivax; antibodies; heterologous; homologous; malaria.
Copyright © 2019 American Society for Microbiology.
Figures


References
-
- Franca CT, Li Wai Suen CSN, Carmagnac A, Lin E, Kiniboro B, Siba P, Schofield L, Mueller I. 2017. IgG antibodies to synthetic GPI are biomarkers of immune-status to both Plasmodium falciparum and Plasmodium vivax malaria in young children. Malar J 16:386. doi: 10.1186/s12936-017-2042-2. - DOI - PMC - PubMed
-
- Cespedes N, Li Wai Suen CSN, Koepfli C, Franca CT, Felger I, Nebie I, Arevalo-Herrera M, Mueller I, Corradin G, Herrera S. 2017. Natural immune response to Plasmodium vivax alpha-helical coiled coil protein motifs and its association with the risk of P. vivax malaria. PLoS One 12:e0179863. doi: 10.1371/journal.pone.0179863. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

