Inhibition of complement C1s improves severe hemolytic anemia in cold agglutinin disease: a first-in-human trial
- PMID: 30559259
- PMCID: PMC6396179
- DOI: 10.1182/blood-2018-06-856930
Inhibition of complement C1s improves severe hemolytic anemia in cold agglutinin disease: a first-in-human trial
Abstract
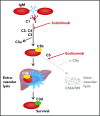
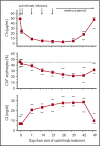
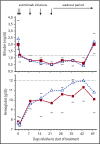
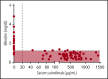
Cold agglutinin disease is a difficult-to-treat autoimmune hemolytic anemia in which immunoglobulin M antibodies bind to erythrocytes and fix complement, resulting in predominantly extravascular hemolysis. This trial tested the hypothesis that the anti-C1s antibody sutimlimab would ameliorate hemolytic anemia. Ten patients with cold agglutinin disease participated in the phase 1b component of a first-in-human trial. Patients received a test dose of 10-mg/kg sutimlimab followed by a full dose of 60 mg/kg 1 to 4 days later and 3 additional weekly doses of 60 mg/kg. All infusions were well tolerated without premedication. No drug-related serious adverse events were observed. Seven of 10 patients with cold agglutinin disease responded with a hemoglobin increase >2 g/dL. Sutimlimab rapidly increased hemoglobin levels by a median of 1.6 g/dL within the first week, and by a median of 3.9 g/dL (interquartile range, 1.3-4.5 g/dL; 95% confidence interval, 2.1-4.5) within 6 weeks (P = .005). Sutimlimab rapidly abrogated extravascular hemolysis, normalizing bilirubin levels within 24 hours in most patients and normalizing haptoglobin levels in 4 patients within 1 week. Hemolytic anemia recurred when drug levels were cleared from the circulation 3 to 4 weeks after the last dose of sutimlimab. Reexposure to sutimlimab in a named patient program recapitulated the control of hemolytic anemia. All 6 previously transfused patients became transfusion-free during treatment. Sutimlimab was safe, well tolerated, and rapidly stopped C1s complement-mediated hemolysis in patients with cold agglutinin disease, significantly increasing hemoglobin levels and precluding the need for transfusions. This trial was registered at www.clinicaltrials.gov as #NCT02502903.
© 2019 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: U.J. reports personal fees from True North Therapeutics during the conduct of the study, grants and personal fees from Roche, grants and personal fees from Celgene, grants and personal fees from Gilead, personal fees from Amgen, grants and personal fees from Novartis, personal fees from Takeda, personal fees from AbbVie, and personal fees from Infinity; outside the submitted work, grants and personal fees from True North Therapeutics. G.P. is a consultant to Bioverativ and was an employee and stockholder of the study sponsor, True North Therapeutics, at the time the study was designed and conducted. G.C.P. is an employee of Bioverativ. S.P. was an employee and stockholder of the study sponsor, True North Therapeutics, at the time the study was designed and conducted, and was subsequently an employee of Bioverativ. J.C.G. was an employee and stockholder of the study sponsor, True North Therapeutics, at the time the study was designed and conducted. B.J. received reimbursement from True North Therapeutics related to travel costs for scientific presentations and scientific advice. The remaining authors declare no competing financial interests.
Figures

Comment in
-
Cold agglutinins: fending off the attack.Blood. 2019 Feb 28;133(9):885-886. doi: 10.1182/blood-2019-01-894303. Blood. 2019. PMID: 30819775 No abstract available.
Similar articles
-
Sutimlimab in Cold Agglutinin Disease.N Engl J Med. 2021 Apr 8;384(14):1323-1334. doi: 10.1056/NEJMoa2027760. N Engl J Med. 2021. PMID: 33826820 Clinical Trial.
-
Inhibition of complement C1s in patients with cold agglutinin disease: lessons learned from a named patient program.Blood Adv. 2020 Mar 24;4(6):997-1005. doi: 10.1182/bloodadvances.2019001321. Blood Adv. 2020. PMID: 32176765 Free PMC article. Clinical Trial.
-
Sutimlimab: A Complement C1s Inhibitor for the Management of Cold Agglutinin Disease-Associated Hemolysis.Ann Pharmacother. 2023 Aug;57(8):970-977. doi: 10.1177/10600280221138802. Epub 2022 Dec 8. Ann Pharmacother. 2023. PMID: 36476151 Review.
-
Sustained inhibition of complement C1s with sutimlimab over 2 years in patients with cold agglutinin disease.Am J Hematol. 2023 Aug;98(8):1246-1253. doi: 10.1002/ajh.26965. Epub 2023 May 29. Am J Hematol. 2023. PMID: 37246953
-
Complement-directed therapy for cold agglutinin disease: sutimlimab.Expert Rev Hematol. 2023 Jul-Dec;16(7):479-494. doi: 10.1080/17474086.2023.2218611. Epub 2023 Jun 2. Expert Rev Hematol. 2023. PMID: 37256550 Review.
Cited by
-
The choice of new treatments in autoimmune hemolytic anemia: how to pick from the basket?Front Immunol. 2023 Apr 24;14:1180509. doi: 10.3389/fimmu.2023.1180509. eCollection 2023. Front Immunol. 2023. PMID: 37168855 Free PMC article. Review.
-
Infectious Complications in Autoimmune Hemolytic Anemia.J Clin Med. 2021 Jan 5;10(1):164. doi: 10.3390/jcm10010164. J Clin Med. 2021. PMID: 33466516 Free PMC article. Review.
-
Complement in neurological disorders and emerging complement-targeted therapeutics.Nat Rev Neurol. 2020 Nov;16(11):601-617. doi: 10.1038/s41582-020-0400-0. Epub 2020 Oct 1. Nat Rev Neurol. 2020. PMID: 33005040 Free PMC article. Review.
-
Quantitative fluorescence resonance energy transfer-based immunoassay for activated complement C1s.Front Immunol. 2023 Jan 24;14:1081793. doi: 10.3389/fimmu.2023.1081793. eCollection 2023. Front Immunol. 2023. PMID: 36761732 Free PMC article.
-
Rituximab Use in Warm and Cold Autoimmune Hemolytic Anemia.J Clin Med. 2020 Dec 13;9(12):4034. doi: 10.3390/jcm9124034. J Clin Med. 2020. PMID: 33322221 Free PMC article. Review.
References
-
- Michel M, Jaeger U. Autoimmune hemolytic anemia. In: Hoffman R, Benz E, Silberstein LE, Heslop H, Weitz J, Anastasi J, eds. Hematology 7th Edition. Basic Principles and Practice. Philadelphia, PA: Elsevier; 2017:648-662.
-
- Pruzanski W, Shumak KH. Biologic activity of cold-reacting autoantibodies (first of two parts). N Engl J Med. 1977;297(10):538-542. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

