Tau impairs neural circuits, dominating amyloid-β effects, in Alzheimer models in vivo
- PMID: 30559471
- PMCID: PMC6560629
- DOI: 10.1038/s41593-018-0289-8
Tau impairs neural circuits, dominating amyloid-β effects, in Alzheimer models in vivo
Abstract
The coexistence of amyloid-β (Aβ) plaques and tau neurofibrillary tangles in the neocortex is linked to neural system failure and cognitive decline in Alzheimer's disease. However, the underlying neuronal mechanisms are unknown. By employing in vivo two-photon Ca2+ imaging of layer 2/3 cortical neurons in mice expressing human Aβ and tau, we reveal a dramatic tau-dependent suppression of activity and silencing of many neurons, which dominates over Aβ-dependent neuronal hyperactivity. We show that neurofibrillary tangles are neither sufficient nor required for the silencing, which instead is dependent on soluble tau. Surprisingly, although rapidly effective in tau mice, suppression of tau gene expression was much less effective in rescuing neuronal impairments in mice containing both Aβ and tau. Together, our results reveal how Aβ and tau synergize to impair the functional integrity of neural circuits in vivo and suggest a possible cellular explanation contributing to disappointing results from anti-Aβ therapeutic trials.
Conflict of interest statement
Competing interests
The authors declare no competing interests.
Figures

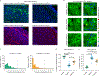
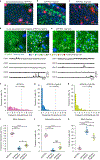
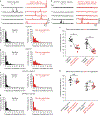
References
-
- Braak H & Braak E Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol 82, 239–259 (1991). - PubMed
-
- Arnold SE, Hyman BT, Flory J, Damasio AR & Van Hoesen GW The topographical and neuroanatomical distribution of neurofibrillary tangles and neuritic plaques in the cerebral cortex of patients with Alzheimer’s disease. Cereb. Cortex 1, 103–116 (1991). - PubMed
-
- Delacourte A et al. The biochemical pathway of neurofibrillary degeneration in aging and Alzheimer’s disease. Neurology 52, 1158–1165 (1999). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

