Need for Speed and Precision: Structural and Functional Specialization in the Cochlear Nucleus of the Avian Auditory System
- PMID: 30559595
- PMCID: PMC6291874
- DOI: 10.1177/1179069518815628
Need for Speed and Precision: Structural and Functional Specialization in the Cochlear Nucleus of the Avian Auditory System
Abstract
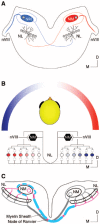
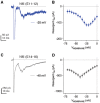
Birds such as the barn owl and zebra finch are known for their remarkable hearing abilities that are critical for survival, communication, and vocal learning functions. A key to achieving these hearing abilities is the speed and precision required for the temporal coding of sound-a process heavily dependent on the structural, synaptic, and intrinsic specializations in the avian auditory brainstem. Here, we review recent work from us and others focusing on the specialization of neurons in the chicken cochlear nucleus magnocellularis (NM)-a first-order auditory brainstem structure analogous to bushy cells in the mammalian anteroventral cochlear nucleus. Similar to their mammalian counterpart, NM neurons are mostly adendritic and receive auditory nerve input through large axosomatic endbulb of Held synapses. Axonal projections from NM neurons to their downstream auditory targets are sophisticatedly programmed regarding their length, caliber, myelination, and conduction velocity. Specialized voltage-dependent potassium and sodium channel properties also play important and unique roles in shaping the functional phenotype of NM neurons. Working synergistically with potassium channels, an atypical current known as resurgent sodium current promotes rapid and precise action potential firing for NM neurons. Interestingly, these structural and functional specializations vary dramatically along the tonotopic axis and suggest a plethora of encoding strategies for sounds of different acoustic frequencies, mechanisms likely shared across species.
Keywords: Auditory; avian; nucleus magnocellularis; potassium channels; resurgent sodium current; sodium channels; tonotopy.
Conflict of interest statement
Declaration of conflicting interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures













Similar articles
-
Resurgent sodium current promotes action potential firing in the avian auditory brainstem.J Physiol. 2018 Feb 1;596(3):423-443. doi: 10.1113/JP275083. Epub 2018 Jan 4. J Physiol. 2018. PMID: 29193076 Free PMC article.
-
Diverse Intrinsic Properties Shape Functional Phenotype of Low-Frequency Neurons in the Auditory Brainstem.Front Cell Neurosci. 2018 Jun 26;12:175. doi: 10.3389/fncel.2018.00175. eCollection 2018. Front Cell Neurosci. 2018. PMID: 29997479 Free PMC article.
-
Developmental Profile of Ion Channel Specializations in the Avian Nucleus Magnocellularis.Front Cell Neurosci. 2016 Mar 30;10:80. doi: 10.3389/fncel.2016.00080. eCollection 2016. Front Cell Neurosci. 2016. PMID: 27065805 Free PMC article.
-
The ion channels and synapses responsible for the physiological diversity of mammalian lower brainstem auditory neurons.Hear Res. 2019 May;376:33-46. doi: 10.1016/j.heares.2018.12.011. Epub 2018 Dec 26. Hear Res. 2019. PMID: 30606624 Review.
-
Regulation of the timing of MNTB neurons by short-term and long-term modulation of potassium channels.Hear Res. 2005 Aug;206(1-2):133-45. doi: 10.1016/j.heares.2004.11.023. Hear Res. 2005. PMID: 16081004 Review.
Cited by
-
Effects of Neurotrophin-3 on Intrinsic Neuronal Properties at a Central Auditory Structure.Neurosci Insights. 2020 Dec 10;15:2633105520980442. doi: 10.1177/2633105520980442. eCollection 2020. Neurosci Insights. 2020. PMID: 33354669 Free PMC article.
-
Electrophysiological correlates of divergent projections in the avian superior olivary nucleus.J Neurophysiol. 2024 Nov 1;132(5):1412-1425. doi: 10.1152/jn.00099.2024. Epub 2024 Sep 11. J Neurophysiol. 2024. PMID: 39258776
-
BDNF Differentially Affects Low- and High-Frequency Neurons in a Primary Nucleus of the Chicken Auditory Brainstem.Biology (Basel). 2024 Oct 29;13(11):877. doi: 10.3390/biology13110877. Biology (Basel). 2024. PMID: 39596832 Free PMC article.
-
ZEBrA: Zebra finch Expression Brain Atlas-A resource for comparative molecular neuroanatomy and brain evolution studies.J Comp Neurol. 2020 Aug;528(12):2099-2131. doi: 10.1002/cne.24879. Epub 2020 Feb 19. J Comp Neurol. 2020. PMID: 32037563 Free PMC article.
-
Slicing the Embryonic Chicken Auditory Brainstem to Evaluate Tonotopic Gradients and Microcircuits.J Vis Exp. 2022 Jul 12;(185):10.3791/63476. doi: 10.3791/63476. J Vis Exp. 2022. PMID: 35913132 Free PMC article.
References
-
- Shannon RV, Zeng FG, Kamath V, Wygonski J, Ekelid M. Speech recognition with primarily temporal cues. Science. 1995;270:303–304. - PubMed
-
- Knudsen EI, Konishi M. Mechanisms of sound localization in the barn owl (Tyto alba). J Comp Physiol. 1979;133:13–21. doi:10.1007/bf00663106. - DOI
-
- Knudsen EI, Blasdel GG, Konishi M. Sound localization by the barn owl (Tyto alba) measured with the search coil technique. J Comp Physiol. 1979;133:1–11. doi:10.1007/bf00663105. - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

