Immune Checkpoint Inhibitors in Pediatric Solid Tumors: Status in 2018
- PMID: 30559623
- PMCID: PMC6292483
- DOI: 10.31486/toj.18.0055
Immune Checkpoint Inhibitors in Pediatric Solid Tumors: Status in 2018
Abstract
Background: Checkpoint inhibitors have transformed the treatment of cancer in adults. This class of drugs has demonstrated encouraging results in various malignancies such as metastatic melanoma, bladder cancer, renal cancer, and non-small cell lung carcinoma. However, researchers have only begun investigating the effectiveness and tolerability of checkpoint inhibitors in pediatric patients.
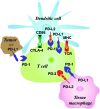
Methods: We conducted a review of PubMed indexed literature and clinicaltrials.gov using combinations of the keywords checkpoint, inhibitor, pediatric, CTLA-4 (cytotoxic T lymphocyte antigen-4), PD-1 (programmed cell death-1), and PD-L1 (programmed cell death receptor-1 ligand) to find every recently completed and ongoing trial evaluating checkpoint inhibitors in patients younger than 21 years old. Pertinent articles and clinical trials discussing the role of immune checkpoint inhibitors in the pediatric population were selected for final analysis and manuscript citation.
Results: This review presents an overview of the cellular mechanisms involved in checkpoint inhibition and of studies evaluating checkpoint inhibitors in humans. The review also details results and side effects from studies conducted with pediatric patients, current pediatric clinical trials, and future implications.
Conclusion: Immune checkpoint inhibitors have the potential to further therapeutic advances in pediatric oncology; however, we need more clinical trials and combination drug strategies targeted toward pediatric cancers.
Keywords: CTLA-4 antigen; Costimulatory and inhibitory T-cell receptors; immune checkpoint inhibitors; immunity–cellular; immunotherapy; programmed cell death 1 receptor.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials