A Ketogenic Diet Improves Cognition and Has Biochemical Effects in Prefrontal Cortex That Are Dissociable From Hippocampus
- PMID: 30559660
- PMCID: PMC6286979
- DOI: 10.3389/fnagi.2018.00391
A Ketogenic Diet Improves Cognition and Has Biochemical Effects in Prefrontal Cortex That Are Dissociable From Hippocampus
Abstract
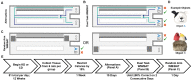
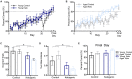
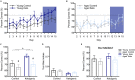
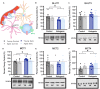
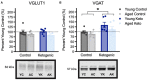
Age-related cognitive decline has been linked to a diverse set of neurobiological mechanisms, including bidirectional changes in proteins critical for neuron function. Importantly, these alterations are not uniform across the brain. For example, the hippocampus (HPC) and prefrontal cortex (PFC) show distinct patterns of dysfunction in advanced age. Because higher cognitive functions require large-scale interactions across prefrontal cortical and hippocampal networks, selectively targeting an alteration within one region may not broadly restore function to improve cognition. One mechanism for decline that the PFC and HPC share, however, is a reduced ability to utilize glucose for energy metabolism. Although this suggests that therapeutic strategies bypassing the need for neuronal glycolysis may be beneficial for treating cognitive aging, this approach has not been empirically tested. Thus, the current study used a ketogenic diet (KD) as a global metabolic strategy for improving brain function in young and aged rats. After 12 weeks, rats were trained to perform a spatial alternation task through an asymmetrical maze, in which one arm was closed and the other was open. Both young and aged KD-fed rats showed resilience against the anxiogenic open arm, training to alternation criterion performance faster than control animals. Following alternation testing, rats were trained to perform a cognitive dual task that required working memory while simultaneously performing a bi-conditional association task (WM/BAT), which requires PFC-HPC interactions. All KD-fed rats also demonstrated improved performance on WM/BAT. At the completion of behavioral testing, tissue punches were collected from the PFC for biochemical analysis. KD-fed rats had biochemical alterations within PFC that were dissociable from previous results in the HPC. Specifically, MCT1 and MCT4, which transport ketone bodies, were significantly increased in KD-fed rats compared to controls. GLUT1, which transports glucose across the blood brain barrier, was decreased in KD-fed rats. Contrary to previous observations within the HPC, the vesicular glutamate transporter (VGLUT1) did not change with age or diet within the PFC. The vesicular GABA transporter (VGAT), however, was increased within PFC similar to HPC. These data suggest that KDs could be optimal for enhancing large-scale network function that is critical for higher cognition.
Keywords: GABA; anxiety; glucose; glutamate; metabolism; monocarboxylate; transporter.
Figures

Similar articles
-
A long-term ketogenic diet in young and aged rats has dissociable effects on prelimbic cortex and CA3 ensemble activity.bioRxiv [Preprint]. 2023 Feb 19:2023.02.18.529095. doi: 10.1101/2023.02.18.529095. bioRxiv. 2023. Update in: Front Aging Neurosci. 2023 Dec 14;15:1274624. doi: 10.3389/fnagi.2023.1274624. PMID: 36824737 Free PMC article. Updated. Preprint.
-
Effects of working memory training on cognitive flexibility, dendritic spine density and long-term potentiation in female mice.Behav Brain Res. 2025 May 28;486:115555. doi: 10.1016/j.bbr.2025.115555. Epub 2025 Mar 26. Behav Brain Res. 2025. PMID: 40154742
-
A long-term ketogenic diet in young and aged rats has dissociable effects on prelimbic cortex and CA3 ensemble activity.Front Aging Neurosci. 2023 Dec 14;15:1274624. doi: 10.3389/fnagi.2023.1274624. eCollection 2023. Front Aging Neurosci. 2023. PMID: 38155737 Free PMC article.
-
Neuromodulation of Hippocampal-Prefrontal Cortical Synaptic Plasticity and Functional Connectivity: Implications for Neuropsychiatric Disorders.Front Cell Neurosci. 2021 Oct 11;15:732360. doi: 10.3389/fncel.2021.732360. eCollection 2021. Front Cell Neurosci. 2021. PMID: 34707481 Free PMC article. Review.
-
Neuromodulation of Prefrontal Cortex in Non-Human Primates by Dopaminergic Receptors during Rule-Guided Flexible Behavior and Cognitive Control.Front Neural Circuits. 2017 Dec 5;11:91. doi: 10.3389/fncir.2017.00091. eCollection 2017. Front Neural Circuits. 2017. PMID: 29259545 Free PMC article. Review.
Cited by
-
Age-related impairments on the touchscreen paired associates learning (PAL) task in male rats.Neurobiol Aging. 2022 Jan;109:176-191. doi: 10.1016/j.neurobiolaging.2021.09.021. Epub 2021 Oct 2. Neurobiol Aging. 2022. PMID: 34749169 Free PMC article.
-
Time restricted feeding with or without ketosis influences metabolism-related gene expression in a tissue-specific manner in aged rats.bioRxiv [Preprint]. 2024 Dec 20:2024.12.19.629431. doi: 10.1101/2024.12.19.629431. bioRxiv. 2024. Update in: Geroscience. 2025 Jun;47(3):4845-4855. doi: 10.1007/s11357-025-01632-7. PMID: 39763909 Free PMC article. Updated. Preprint.
-
Perspective: Ketone Supplementation in Sports-Does It Work?Adv Nutr. 2021 Mar 31;12(2):305-315. doi: 10.1093/advances/nmaa130. Adv Nutr. 2021. PMID: 33094332 Free PMC article.
-
Age-Related Alterations in Prelimbic Cortical Neuron Arc Expression Vary by Behavioral State and Cortical Layer.Front Aging Neurosci. 2020 Oct 28;12:588297. doi: 10.3389/fnagi.2020.588297. eCollection 2020. Front Aging Neurosci. 2020. PMID: 33192482 Free PMC article.
-
Are ketogenic diets promising for Alzheimer's disease? A translational review.Alzheimers Res Ther. 2020 Apr 14;12(1):42. doi: 10.1186/s13195-020-00615-4. Alzheimers Res Ther. 2020. PMID: 32290868 Free PMC article.
References
-
- Anderson K. L., Frazier H. N., Maimaiti S., Bakshi V. V., Majeed Z. R., Brewer L. D., et al. (2017). Impact of single or repeated dose intranasal zinc-free insulin in young and aged F344 rats on cognition, signaling, and brain metabolism. J. Gerontol. A Biol. Sci. Med. Sci. 72 189–197. 10.1093/gerona/glw065 - DOI - PMC - PubMed
-
- Bailey K. R., Crawley J. N. (2009). “Anxiety-related behaviors in mice,” in Methods of Behavior Analysis in Neuroscience Frontiers in Neuroscience, ed. Buccafusco J. J. (Boca Raton, FL: CRC Press/Taylor & Francis; ). - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

