Disease Tolerance and Pathogen Resistance Genes May Underlie Trypanosoma cruzi Persistence and Differential Progression to Chagas Disease Cardiomyopathy
- PMID: 30559742
- PMCID: PMC6286977
- DOI: 10.3389/fimmu.2018.02791
Disease Tolerance and Pathogen Resistance Genes May Underlie Trypanosoma cruzi Persistence and Differential Progression to Chagas Disease Cardiomyopathy
Abstract
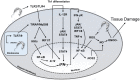
Chagas disease is caused by infection with the protozoan Trypanosoma cruzi and affects over 8 million people worldwide. In spite of a powerful innate and adaptive immune response in acute infection, the parasite evades eradication, leading to a chronic persistent infection with low parasitism. Chronically infected subjects display differential patterns of disease progression. While 30% develop chronic Chagas disease cardiomyopathy (CCC)-a severe inflammatory dilated cardiomyopathy-decades after infection, 60% of the patients remain disease-free, in the asymptomatic/indeterminate (ASY) form, and 10% develop gastrointestinal disease. Infection of genetically deficient mice provided a map of genes relevant for resistance to T. cruzi infection, leading to the identification of multiple genes linked to survival to infection. These include pathogen resistance genes (PRG) needed for intracellular parasite destruction, and genes involved in disease tolerance (protection against tissue damage and acute phase death-DTG). All identified DTGs were found to directly or indirectly inhibit IFN-γ production or Th1 differentiation. We hypothesize that the absolute need for DTG to control potentially lethal IFN-γ PRG activity leads to T. cruzi persistence and establishment of chronic infection. IFN-γ production is higher in CCC than ASY patients, and is the most highly expressed cytokine in CCC hearts. Key DTGs that downmodulate IFN-γ, like IL-10, and Ebi3/IL27p28, are higher in ASY patients. Polymorphisms in PRG and DTG are associated with differential disease progression. We thus hypothesize that ASY patients are disease tolerant, while an imbalance of DTG and IFN-γ PRG activity leads to the inflammatory heart damage of CCC.
Keywords: Tripanosoma cruzi; chagas cardiomyopathy; disease tolerance gene; interferon gamma; pathogen resistance genes.
Figures



References
-
- Harris LD, Tabb B, Sodora DL, Paiardini M, Klatt NR, Douek DC, et al. Downregulation of robust acute type I interferon responses distinguishes nonpathogenic simian immunodeficiency virus (SIV) infection of natural hosts from pathogenic SIV infection of rhesus macaques. J Virol. (2010) 84:7886–91. 10.1128/JVI.02612-09 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

