The Adhesion G Protein-Coupled Receptor GPR97/ ADGRG3 Is Expressed in Human Granulocytes and Triggers Antimicrobial Effector Functions
- PMID: 30559745
- PMCID: PMC6287011
- DOI: 10.3389/fimmu.2018.02830
The Adhesion G Protein-Coupled Receptor GPR97/ ADGRG3 Is Expressed in Human Granulocytes and Triggers Antimicrobial Effector Functions
Abstract
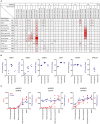
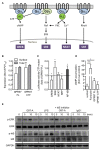
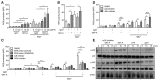
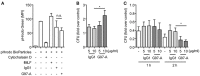
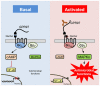
The adhesion family of G protein-coupled receptors (aGPCRs) comprises 33 members in human, several of which are distinctly expressed and functionally involved in polymorphonuclear cells (PMNs). As former work indicated the possible presence of the aGPCR GPR97 in granulocytes, we studied its cellular distribution, molecular structure, signal transduction, and biological function in PMNs. RNA sequencing and mass-spectrometry revealed abundant RNA and protein expression of ADGRG3/GPR97 in granulocyte precursors and terminally differentiated neutrophilic, eosinophilic, and basophilic granulocytes. Using a newly generated GPR97-specific monoclonal antibody, we confirmed that endogenous GPR97 is a proteolytically processed, dichotomous, N-glycosylated receptor. GPR97 was detected in tissue-infiltrating PMNs and upregulated during systemic inflammation. Antibody ligation of GPR97 increased neutrophil reactive oxygen species production and proteolytic enzyme activity, which is accompanied by an increase in mitogen-activated protein kinases and IκBα phosphorylation. In-depth analysis of the GPR97 signaling cascade revealed a possible switch from basal Gαs/cAMP-mediated signal transduction to a Gαi-induced reduction in cAMP levels upon mutation-induced activation of the receptor, in combination with an increase in downstream effectors of Gβγ, such as SRE and NF-κB. Finally, ligation of GPR97 increased the bacteria uptake and killing activity of neutrophils. We conclude that the specific presence of GPR97 regulates antimicrobial activity in human granulocytes.
Keywords: G-protein signaling; adhesion GPCR; antimicrobial activity; granulocytes; inflammation; monoclonal antibody.
Figures



References
-
- Janeway CA, Traver P, Walport M, Shlomchik MJ. Immunobiology, 5th Edn. New York, NY: Garland Science; (2001). Available online at: https://www.ncbi.nlm.nih.gov/books/NBK10757/
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

