Autonomic responses to blast overpressure can be elicited by exclusively exposing the ear in rats
- PMID: 30559764
- PMCID: PMC6291641
- DOI: 10.1016/j.joto.2018.01.001
Autonomic responses to blast overpressure can be elicited by exclusively exposing the ear in rats
Erratum in
-
Erratum regarding missing Declaration of Competing Interest statements in previously published articles.J Otol. 2020 Dec;15(4):178. doi: 10.1016/j.joto.2020.09.005. Epub 2020 Sep 26. J Otol. 2020. PMID: 33293922 Free PMC article.
Abstract
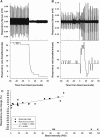
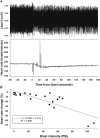
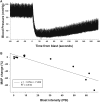
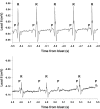
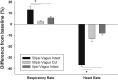
Blast overpressure has become an increasing cause of brain injuries in both military and civilian populations. Though blast's direct effects on the cochlea and vestibular organs are active areas of study, little attention has been given to the ear's contribution to the overall spectrum of blast injury. Acute autonomic responses to blast exposure, including bradycardia and hypotension, can cause hypoxia and contribute to blast-induced neurotrauma. Existing literature suggests that these autonomic responses are elicited through blast impacting the thorax and lungs. We hypothesize that the unprotected ear also provides a vulnerable locus for blast to cause autonomic responses. We designed a blast generator that delivers controlled overpressure waves into the ear canal without impacting surrounding tissues in order to study the ear's specific contribution to blast injury. Anesthetized adult rats' left ears were exposed to a single blast wave ranging from 0 to 110 PSI (0-758 kPa). Blast exposed rats exhibited decreased heart rates and blood pressures with increased blast intensity, similar to results gathered using shock tubes and whole-body exposure in the literature. While rats exposed to blasts below 50 PSI (345 kPa) exhibited increased respiratory rate with increased blast intensity, some rats exposed to blasts higher than 50 PSI (345 kPa) stopped breathing immediately and ultimately died. These autonomic responses were significantly reduced in vagally denervated rats, again similar to whole-body exposure literature. These results support the hypothesis that the unprotected ear contributes to the autonomic responses to blast.
Keywords: Autonomic responses; Blast waves; Ear; Neurotrauma.
Figures

References
-
- Abel S.M. Barriers to hearing conservation programs in combat arms occupations. Aviat Space Environ. Med. 2008;79:591–598. - PubMed
-
- Akin F.W., Murnane O.D. Head injury and blast exposure: vestibular consequences. Otolaryngol. Clin. North Am. 2011 - PubMed
-
- Alay E., Skotak M., Misistia A., Chandra N. Dynamic loads on human and animal surrogates at different test locations in compressed-gas-driven shock tubes. Shock Waves. 2017:1–12.
-
- Bass C.R., Panzer M.B., Rafaels K.A., Wood G., Shridharani J., Capehart B. Brain injuries from blast. Ann. Biomed. Eng. 2012;40:185–202. - PubMed
-
- Berthoud H.-R., Neuhuber W.L. Functional and chemical anatomy of the afferent vagal system. Auton. Neurosci. 2000;85:1–17. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

