Perioperative administration of buffered versus non-buffered crystalloid intravenous fluid to improve outcomes following adult surgical procedures: a Cochrane systematic review
- PMID: 30559961
- PMCID: PMC6291967
- DOI: 10.1186/s13741-018-0108-5
Perioperative administration of buffered versus non-buffered crystalloid intravenous fluid to improve outcomes following adult surgical procedures: a Cochrane systematic review
Abstract
Background: Buffered intravenous fluid preparations contain substrates to maintain acid-base status. The objective of this systematic review was to compare the effects of buffered and non-buffered fluids administered during the perioperative period on clinical and biochemical outcomes.
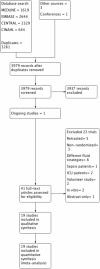
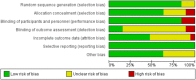
Methods: We searched MEDLINE, EMBASE, CINAHL and the Cochrane Library until May 2017 and included all randomised controlled trials that evaluated buffered versus non-buffered fluids, whether crystalloid or colloid, administered to surgical patients. We assessed the selected studies for risk of bias and graded the level of evidence in accordance with Cochrane recommendations.
Results: We identified 19 publications of 18 randomised controlled trials, totalling 1096 participants. Mean difference (MD) in postoperative pH was 0.05 units lower immediately following surgery in the non-buffered group (12 studies of 720 participants; 95% confidence interval (CI) 0.04 to 0.07; I 2 = 61%). This difference did not persist on postoperative day 1. Serum chloride concentration was higher in the non-buffered group at the end of surgery (10 trials of 530 participants; MD 6.77 mmol/L, 95% CI 3.38 to 10.17). This effect persisted until postoperative day 1 (5 trials of 258 participants; MD 8.48 mmol/L, 95% CI 1.08 to 15.88). Quality of this evidence was moderate. We identified variable protocols for fluid administration and total volumes of fluid administered to patients intraoperatively. Outcome data was variably reported at disparate time points and with heterogeneous patient groups. Consequently, the effect size and overall confidence interval was reduced, despite the relatively low inherent risk of bias. There was insufficient evidence on the effect of fluid composition on mortality and organ dysfunction. Confidence intervals of this outcome were wide and the quality of evidence was low (3 trials of 276 participants for mortality; odds ratio (OR) 1.85, 95% CI 0.37 to 9.33; I 2 = 0%).
Conclusions: Small effect sizes for biochemical outcomes and lack of correlated clinical follow-up data mean that robust conclusions on major morbidity and mortality associated with buffered versus non-buffered perioperative fluid choices are still lacking. Buffered fluid may have biochemical benefits, including a significant reduction in postoperative hyperchloraemia and metabolic acidosis.
Keywords: Fluid therapy; Plasma substitutes; Surgery.
Conflict of interest statement
Not applicableNot applicableAuthors of this review (Elliott Bennett-Guerrero, Tong J Gan, Michael G Mythen, Catherine O’Malley) authored five of the primary studies included in this review. Michael FM James has received lecture support and honoraria from Fresenius Kabi. Peter M Odor and Sohail Bampoe, who were not authors of any primary studies, extracted data from all studies in this review. None of the remaining authors have any conflicts of interest to declare.Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
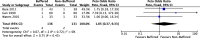


References
-
- Calvo-Vecino JM, Ripollés-Melchor J, Mythen MG, Casans-Francés R, Balik A, Artacho JP, et al. Effect of goal-directed haemodynamic therapy on postoperative complications in low–moderate risk surgical patients: a multicentre randomised controlled trial (FEDORA trial) Br J Anaesth. 2018;120(4):734–744. doi: 10.1016/j.bja.2017.12.018. - DOI - PubMed
-
- Chin J, Macachor J, Ong KC, Ong BC. A comparison of 5% dextrose in 0.9% normal saline versus non-dextrose- containing crystalloids as the initial intravenous replacement fluid in elective surgery. Anaesth Intensive Care. 2006;34(5):613–617. - PubMed
-
- Gan TJ, Bennett-Guerrero E, Phillips-Bute B, Wakeling H, Moskowitz DM, Olufolabi Y, et al. Hextend, a physiologically balanced plasma expander for large volume use in major surgery: a randomized phase III clinical trial. Anesth Analg. 1999;88(5):992–998. doi: 10.1213/00000539-199905000-00005. - DOI - PubMed

