Manipulating Eryptosis of Human Red Blood Cells: A Novel Antimalarial Strategy?
- PMID: 30560094
- PMCID: PMC6284368
- DOI: 10.3389/fcimb.2018.00419
Manipulating Eryptosis of Human Red Blood Cells: A Novel Antimalarial Strategy?
Erratum in
-
Erratum: Manipulating Eryptosis of Human Red Blood Cells: A Novel Antimalarial Strategy?Front Cell Infect Microbiol. 2019 Jan 14;8:455. doi: 10.3389/fcimb.2018.00455. eCollection 2018. Front Cell Infect Microbiol. 2019. PMID: 30693274 Free PMC article.
Abstract
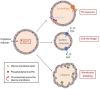
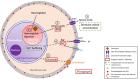
Malaria is a major global health burden, affecting over 200 million people worldwide. Resistance against all currently available antimalarial drugs is a growing threat, and represents a major and long-standing obstacle to malaria eradication. Like many intracellular pathogens, Plasmodium parasites manipulate host cell signaling pathways, in particular programmed cell death pathways. Interference with apoptotic pathways by malaria parasites is documented in the mosquito and human liver stages of infection, but little is known about this phenomenon in the erythrocytic stages. Although mature erythrocytes have lost all organelles, they display a form of programmed cell death termed eryptosis. Numerous features of eryptosis resemble those of nucleated cell apoptosis, including surface exposure of phosphatidylserine, cell shrinkage and membrane ruffling. Upon invasion, Plasmodium parasites induce significant stress to the host erythrocyte, while delaying the onset of eryptosis. Many eryptotic inducers appear to have a beneficial effect on the course of malaria infection in murine models, but major gaps remain in our understanding of the underlying molecular mechanisms. All currently available antimalarial drugs have parasite-encoded targets, which facilitates the emergence of resistance through selection of mutations that prevent drug-target binding. Identifying host cell factors that play a key role in parasite survival will provide new perspectives for host-directed anti-malarial chemotherapy. This review focuses on the interrelationship between Plasmodium falciparum and the eryptosis of its host erythrocyte. We summarize the current knowledge in this area, highlight the different schools of thoughts and existing gaps in knowledge, and discuss future perspectives for host-directed therapies in the context of antimalarial drug discovery.
Keywords: Plasmodium; apoptosis; eryptosis; host-directed therapy; host-pathogen interaction; malaria; programmed cell death.
Figures

References
-
- Adovelande J., Bastide B., Délèze J., Schrével J. (1993). Cytosolic free calcium in Plasmodium falciparum-infected erythrocytes and the effect of verapamil: a cytofluorimetric study. Exp. Parasitol. 76, 247–258. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

