Examination of a paradox: recurrent metastatic breast cancer incidence decline without improved distant disease survival: 1990-2011
- PMID: 30560462
- PMCID: PMC6422972
- DOI: 10.1007/s10549-018-05090-y
Examination of a paradox: recurrent metastatic breast cancer incidence decline without improved distant disease survival: 1990-2011
Abstract
Purpose: Distant relapse metastatic breast cancer (rMBC) incidence and survival are vital measures of breast cancer diagnosis and treatment progress over time.
Methods: We conducted a retrospective cohort study of stage I-III invasive breast cancer, 1990-2011, follow-up through 2016 [N = 8292, rMBC = 964 (12%)] at a community-based institution. Patient and tumor characteristics (treatment, distant recurrence, vital status) from BC registry data were evaluated. Survival analysis and Cox proportional hazards (HzR) with 95% confidence intervals (95% CI) were calculated using distant recurrence and distant disease-specific survival (DDSS) endpoints.
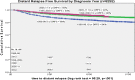
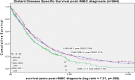
Results: Both 5- and 10-year distant relapse (rMBC) declined over time from 1990-1998 to 2005-2011 [11% to 5%, 16% to 8% (p < 0.001)]. Proportionately, HER2 + BC distant relapse decreased 9% and triple negative (HR-/HER2-) increased 8% (p = 0.011). In the Cox model, lower stage [stage I: HzR = 0.08 (0.07, 0.10), stage II: 0.29 (0.25, 0.33)], more recent diagnosis years [1999-2004: HzR = 0.60 (0.51, 0.70), 2005-2011: HzR = 0.44 (0.38, 0.52)], HR+ [HzR = 0.62 (0.53, 0.72)], and age 40+ [HzR = 0.81 (0.67, 0.98)] had decreased rMBC risk. Compared to HR+/HER2- BC, triple-negative BC had increased rMBC risk [HzR = 2.02 (1.61, 2.53)] but HER2+ subtypes did not. HR-, age 70+, > 1, or visceral metastases and stage III disease were associated with worse DDSS. DDSS did not improve over time.
Conclusion: rMBC incidence declined over time with decreased HER2-positive distant recurrence, a shift to more triple-negative BC and consistently poor distant disease survival.
Keywords: Breast cancer; Distant relapse-free survival; Incidence; Metastatic breast cancer; Risk modeling.
Conflict of interest statement
Conflict of interest
The authors have no conflicts of interest to report.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. This study was IRB approved.
Figures
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous