Targeted Systemic Treatment of Neuroendocrine Tumors: Current Options and Future Perspectives
- PMID: 30560479
- PMCID: PMC6338796
- DOI: 10.1007/s40265-018-1033-0
Targeted Systemic Treatment of Neuroendocrine Tumors: Current Options and Future Perspectives
Abstract
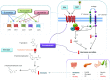
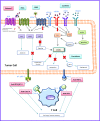
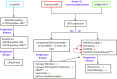
Neuroendocrine tumors (NETs) originate from the neuroendocrine cell system in the bronchial and gastrointestinal tract and can produce hormones leading to distinct clinical syndromes. Systemic treatment of patients with unresectable NETs aims to control symptoms related to hormonal overproduction and tumor growth. In the last decades prognosis has improved as a result of increased detection of early stage disease and the introduction of somatostatin analogs (SSAs) as well as several new therapeutic options. SSAs are the first-line medical treatment of NETs and can control hormonal production and tumor growth. The development of next-generation multireceptor targeted and radiolabelled somatostatin analogs, as well as target-directed therapies (as second-line treatment options) further improve progression-free survival in NET patients. To date, however, a significant prolongation of overall survival with systemic treatment in NET has not been convincingly demonstrated. Several new medical options and treatment combinations will become available in the upcoming years, and although preliminary results of preclinical and clinical trials are encouraging, large, preferrably randomized clinical studies are required to provide definitive evidence of their effect on survival and symptom control.
Conflict of interest statement
Justo P. Castaño has received travel or speaker fees from Novartis/Ipsen and research funds from Ipsen/Novartis. Johannes Hofland has received travel or speaker fees from Ipsen, Novartis, and Advanced Accelerator Applications, research funds from Ipsen, and is on the Advisory Boards of Novartis. Wouter W. de Herder has received travel or speaker fees from Novartis and Ipsen, and research funds from Ipsen. Leo J. Hofland has received research funds from Ipsen and Novartis. Aura D. Herrera-Martínez, Tessa Brabander, Ferry A.L.M. Eskens, María A. Gálvez Moreno, Raúl M. Luque, and Richard A. Feelders declare that they have no conflicts of interest that might be relevant to the contents of this manuscript.
Figures

References
-
- Ameri P, Ferone D. Diffuse endocrine system, neuroendocrine tumors and immunity: what’s new? Neuroendocrinology. 2012;95(4):267–276. - PubMed
-
- Rosai J. The origin of neuroendocrine tumors and the neural crest saga. Mod Pathol. 2011;24(Suppl 2):S53–S57. - PubMed
-
- Fraenkel M, et al. Incidence of gastroenteropancreatic neuroendocrine tumours: a systematic review of the literature. Endocr Relat Cancer. 2014;21(3):R153–R163. - PubMed
-
- Kunz PL. Carcinoid and neuroendocrine tumors: building on success. J Clin Oncol. 2015;33(16):1855–1863. - PubMed
-
- Kim JY, Hong SM, Ro JY. Recent updates on grading and classification of neuroendocrine tumors. Ann Diagn Pathol. 2017;29:11–16. - PubMed

