Hidden heterogeneity and circadian-controlled cell fate inferred from single cell lineages
- PMID: 30560953
- PMCID: PMC6299096
- DOI: 10.1038/s41467-018-07788-5
Hidden heterogeneity and circadian-controlled cell fate inferred from single cell lineages
Abstract
The origin of lineage correlations among single cells and the extent of heterogeneity in their intermitotic times (IMT) and apoptosis times (AT) remain incompletely understood. Here we developed single cell lineage-tracking experiments and computational algorithms to uncover correlations and heterogeneity in the IMT and AT of a colon cancer cell line before and during cisplatin treatment. These correlations could not be explained using simple protein production/degradation models. Sister cell fates were similar regardless of whether they divided before or after cisplatin administration and did not arise from proximity-related factors, suggesting fate determination early in a cell's lifetime. Based on these findings, we developed a theoretical model explaining how the observed correlation structure can arise from oscillatory mechanisms underlying cell fate control. Our model recapitulated the data only with very specific oscillation periods that fit measured circadian rhythms, thereby suggesting an important role of the circadian clock in controlling cellular fates.
Conflict of interest statement
The authors declare no competing interests.
Figures

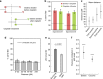




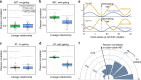
References
-
- Milestones in cell division. Nat. Cell Biol. 3, E265–E265 (2001). - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

