Risk of Death Following Application of Paclitaxel-Coated Balloons and Stents in the Femoropopliteal Artery of the Leg: A Systematic Review and Meta-Analysis of Randomized Controlled Trials
- PMID: 30561254
- PMCID: PMC6405619
- DOI: 10.1161/JAHA.118.011245
Risk of Death Following Application of Paclitaxel-Coated Balloons and Stents in the Femoropopliteal Artery of the Leg: A Systematic Review and Meta-Analysis of Randomized Controlled Trials
Abstract
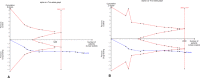
Background Several randomized controlled trials ( RCT s) have already shown that paclitaxel-coated balloons and stents significantly reduce the rates of vessel restenosis and target lesion revascularization after lower extremity interventions. Methods and Results A systematic review and meta-analysis of RCT s investigating paclitaxel-coated devices in the femoral and/or popliteal arteries was performed. The primary safety measure was all-cause patient death. Risk ratios and risk differences were pooled with a random effects model. In all, 28 RCT s with 4663 patients (89% intermittent claudication) were analyzed. All-cause patient death at 1 year (28 RCT s with 4432 cases) was similar between paclitaxel-coated devices and control arms (2.3% versus 2.3% crude risk of death; risk ratio, 1.08; 95% CI, 0.72-1.61). All-cause death at 2 years (12 RCT s with 2316 cases) was significantly increased in the case of paclitaxel versus control (7.2% versus 3.8% crude risk of death; risk ratio, 1.68; 95% CI, 1.15-2.47; -number-needed-to-harm, 29 patients [95% CI , 19-59]). All-cause death up to 5 years (3 RCT s with 863 cases) increased further in the case of paclitaxel (14.7% versus 8.1% crude risk of death; risk ratio, 1.93; 95% CI , 1.27-2.93; -number-needed-to-harm, 14 patients [95% CI , 9-32]). Meta-regression showed a significant relationship between exposure to paclitaxel (dose-time product) and absolute risk of death (0.4±0.1% excess risk of death per paclitaxel mg-year; P<0.001). Trial sequential analysis excluded false-positive findings with 99% certainty (2-sided α, 1.0%). Conclusions There is increased risk of death following application of paclitaxel-coated balloons and stents in the femoropopliteal artery of the lower limbs. Further investigations are urgently warranted. Clinical Trial Registration URL : www.crd.york.ac.uk/PROSPERO . Unique identifier: CRD 42018099447.
Keywords: balloon angioplasty; paclitaxel; paclitaxel‐coated balloon; paclitaxel‐eluting stent.
Figures


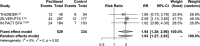

Comment in
-
The Use of Drug Coated Balloon in Femoro-popliteal Atherosclerotic Disease.Eur J Vasc Endovasc Surg. 2019 Sep;58(3):467. doi: 10.1016/j.ejvs.2018.10.022. Epub 2018 Dec 3. Eur J Vasc Endovasc Surg. 2019. PMID: 30522948 No abstract available.
-
Mortality risk of femoropopliteal paclitaxel-coated devices remains inconclusive.Cardiovasc Interv Ther. 2019 Apr;34(2):194-195. doi: 10.1007/s12928-019-00573-1. Epub 2019 Feb 8. Cardiovasc Interv Ther. 2019. PMID: 30737654 Free PMC article. No abstract available.
-
Use of Paclitaxel-Eluting Technologies in the Femoropopliteal Segment Under Scrutiny Over Possible Link to Late All-Cause Mortality: Time to Panic or an Opportunity to Resurge?J Endovasc Ther. 2019 Feb;26(1):41-43. doi: 10.1177/1526602818824682. J Endovasc Ther. 2019. PMID: 30760131 No abstract available.
-
Response to Letter by Bonassi on Article, "Risk of Death Following Application of Paclitaxel-Coated Balloons and Stents in the Femoropopliteal Artery of the Leg: A Systematic Review and Meta-Analysis of Randomized Controlled Trials".J Am Heart Assoc. 2019 May 21;8(10):e012172. doi: 10.1161/JAHA.119.012172. J Am Heart Assoc. 2019. PMID: 31094263 Free PMC article. No abstract available.
-
Is Paclitaxel Causing Mortality During Lower-Extremity Revascularization?J Am Heart Assoc. 2019 May 21;8(10):e012523. doi: 10.1161/JAHA.119.012523. J Am Heart Assoc. 2019. PMID: 31094266 Free PMC article. No abstract available.
-
Letter by Bonassi Regarding Article, "Risk of Death Following Application of Paclitaxel-Coated Balloons and Stents in the Femoropopliteal Artery of the Leg: A Systematic Review and Meta-Analysis of Randomized Controlled Trials".J Am Heart Assoc. 2019 May 21;8(10):e012315. doi: 10.1161/JAHA.119.012315. J Am Heart Assoc. 2019. PMID: 31094268 Free PMC article. No abstract available.
-
Is There a Real Association Between Paclitaxel Devices and Mortality? Time to Pause and Re-Evaluate What We Know About This Statistical Finding.J Am Heart Assoc. 2019 May 21;8(10):e012524. doi: 10.1161/JAHA.119.012524. J Am Heart Assoc. 2019. PMID: 31094276 Free PMC article. No abstract available.
-
Pushing Pause on the Paclitaxel Debate: Applying Appropriate Scientific Inquiry and Publication Processes to Inform Clinical Decision-Making.J Am Coll Cardiol. 2019 Jun 4;73(21):2775-2779. doi: 10.1016/j.jacc.2019.04.018. J Am Coll Cardiol. 2019. PMID: 31146823 No abstract available.
-
Paclitaxel and Mortality: The Dose Argument Is Critical.J Endovasc Ther. 2019 Aug;26(4):467-470. doi: 10.1177/1526602819857241. Epub 2019 Jun 9. J Endovasc Ther. 2019. PMID: 31179816 No abstract available.
-
Bursting the drug-eluting balloon bubble for femoropopliteal disease might be premature.J Vasc Surg. 2019 Jul;70(1):1-2. doi: 10.1016/j.jvs.2019.04.468. J Vasc Surg. 2019. PMID: 31230642 No abstract available.
-
Commentary on Drug-Eluting Technologies.Cardiovasc Intervent Radiol. 2019 Oct;42(10):1508-1509. doi: 10.1007/s00270-019-02297-4. Epub 2019 Aug 5. Cardiovasc Intervent Radiol. 2019. PMID: 31385005 No abstract available.
-
What Now for the Endovascular Community After the Paclitaxel Mortality Meta-Analysis: Can Sirolimus Replace Paclitaxel in the Peripheral Vasculature?J Endovasc Ther. 2020 Feb;27(1):153-156. doi: 10.1177/1526602819881156. Epub 2019 Oct 14. J Endovasc Ther. 2020. PMID: 31608741 No abstract available.
-
The hypothesis of an increased mortality following paclitaxel coated device use in peripheral vascular interventions (and the emerging era of meta-analysis based evidence).Catheter Cardiovasc Interv. 2020 Feb;95(2):329-331. doi: 10.1002/ccd.28600. Epub 2019 Nov 19. Catheter Cardiovasc Interv. 2020. PMID: 31743534 No abstract available.
-
False Alarm! Let's Not Disadvantage Dialysis Patients by Inappropriately Applying Concerns About Paclitaxel-Coated Devices.J Endovasc Ther. 2020 Feb;27(1):163. doi: 10.1177/1526602819896004. J Endovasc Ther. 2020. PMID: 31948373 No abstract available.
-
Fragility of the Signal.J Endovasc Ther. 2020 Oct;27(5):871-872. doi: 10.1177/1526602820941800. Epub 2020 Jul 15. J Endovasc Ther. 2020. PMID: 32666870 No abstract available.
-
No Increased Mortality Risk Following Paclitaxel Treatment in a Large Swedish Registry Based Randomised Controlled Trial - Reassuring Patient Safety.Eur J Vasc Endovasc Surg. 2021 Jan;61(1):5-6. doi: 10.1016/j.ejvs.2020.12.003. Eur J Vasc Endovasc Surg. 2021. PMID: 33451468 No abstract available.
-
Utility of Sirolimus Coated Balloons for Salvaging Dysfunctional Arteriovenous Fistulae: One Year Results From the MATILDA trial.Eur J Vasc Endovasc Surg. 2021 Aug;62(2):316-317. doi: 10.1016/j.ejvs.2021.04.014. Epub 2021 Jun 4. Eur J Vasc Endovasc Surg. 2021. PMID: 34099380 No abstract available.
-
Prevention of neointimal hyperplasia using drug-eluting devices.J Vasc Surg. 2021 Aug;74(2):487-488. doi: 10.1016/j.jvs.2021.02.003. J Vasc Surg. 2021. PMID: 34303475 No abstract available.
-
Midterm Mortality between Single or Multiple Exposure to Paclitaxel Coated Devices for the Treatment of Femoropopliteal Artery Disease.Eur J Vasc Endovasc Surg. 2022 Mar;63(3):521-522. doi: 10.1016/j.ejvs.2021.09.027. Epub 2021 Nov 24. Eur J Vasc Endovasc Surg. 2022. PMID: 34836789 No abstract available.
-
Paclitaxel-coated balloons are safe for the treatment of arterial stenoses.Lancet. 2023 Nov 18;402(10415):1808-1809. doi: 10.1016/S0140-6736(23)02300-0. Epub 2023 Oct 24. Lancet. 2023. PMID: 37890500 No abstract available.
References
-
- Gerhard‐Herman MD, Gornik HL, Barrett C, Barshes NR, Corriere MA, Drachman DE, Fleisher LA, Fowkes FG, Hamburg NM, Kinlay S, Lookstein R, Misra S, Mureebe L, Olin JW, Patel RA, Regensteiner JG, Schanzer A, Shishehbor MH, Stewart KJ, Treat‐Jacobson D, Walsh ME. 2016 AHA/ACC guideline on the management of patients with lower extremity peripheral artery disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2017;135:e686–e725. - PMC - PubMed
-
- Olin JW, Allie DE, Belkin M, Bonow RO, Casey DE Jr, Creager MA, Gerber TC, Hirsch AT, Jaff MR, Kaufman JA, Lewis CA, Martin ET, Martin LG, Sheehan P, Stewart KJ, Treat‐Jacobson D, White CJ, Zheng ZJ, Masoudi FA, Bonow RO, DeLong E, Erwin JP III, Goff DC Jr, Grady K, Green LA, Heidenreich PA, Jenkins KJ, Loth AR, Peterson ED, Shahian DM; American College of Cardiology Foundation; American Heart Association; American College of Radiology; Society for Cardiac Angiography Interventions; Society for Interventional Radiology; Society for Vascular Medicine; Society for Vascular Nursing; Society for Vascular Surgery . ACCF/AHA/ACR/SCAI/SIR/SVM/SVN/SVS 2010 performance measures for adults with peripheral artery disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Performance Measures, the American College of Radiology, the Society for Cardiac Angiography and Interventions, the Society for Interventional Radiology, the Society for Vascular Medicine, the Society for Vascular Nursing, and the Society for Vascular Surgery (Writing Committee to Develop Clinical Performance Measures for Peripheral Artery Disease). J Am Coll Cardiol. 2010;56:2147–2181. - PubMed
-
- Katsanos K, Tepe G, Tsetis D, Fanelli F. Standards of practice for superficial femoral and popliteal artery angioplasty and stenting. Cardiovasc Intervent Radiol. 2014;37:592–603. - PubMed
-
- Katsanos K, Spiliopoulos S, Karunanithy N, Krokidis M, Sabharwal T, Taylor P. Bayesian network meta‐analysis of nitinol stents, covered stents, drug‐eluting stents, and drug‐coated balloons in the femoropopliteal artery. J Vasc Surg. 2014;59:1123–1133.e8. - PubMed
-
- Ng VG, Mena C, Pietras C, Lansky AJ. Local delivery of paclitaxel in the treatment of peripheral arterial disease. Eur J Clin Invest. 2015;45:333–345. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

