Metabolomic Fingerprinting of Infants Undergoing Cardiopulmonary Bypass: Changes in Metabolic Pathways and Association With Mortality and Cardiac Intensive Care Unit Length of Stay
- PMID: 30561257
- PMCID: PMC6405618
- DOI: 10.1161/JAHA.118.010711
Metabolomic Fingerprinting of Infants Undergoing Cardiopulmonary Bypass: Changes in Metabolic Pathways and Association With Mortality and Cardiac Intensive Care Unit Length of Stay
Abstract
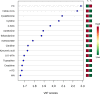
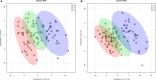
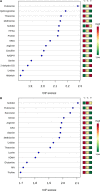
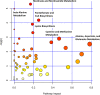
Background Mortality for infants undergoing complex cardiac surgery is >10% with a 30% to 40% risk of complications. Early identification and treatment of high-risk infants remains challenging. Metabolites are small molecules that determine the minute-to-minute cellular phenotype, making them ideal biomarkers for postsurgical monitoring and potential targets for intervention. Methods and Results We measured 165 serum metabolites by tandem mass spectroscopy in infants ≤120 days old undergoing cardiopulmonary bypass. Samples were collected prebypass, during rewarming, and 24 hours after surgery. Partial least squares-discriminant analysis, pathway analysis, and receiver operator characteristic curve analysis were used to evaluate changes in the metabolome, assess altered metabolic pathways, and discriminate between survivors/nonsurvivors as well as upper/lower 50% intensive care unit length of stay. Eighty-two infants had preoperative samples for analysis; 57 also had rewarming and 24-hour samples. Preoperation, the metabolic fingerprint of neonates differed from older infants ( R2=0.89, Q2=0.77; P<0.001). Cardiopulmonary bypass resulted in progressive, age-independent metabolic disturbance ( R2=0.92, Q2=0.83; P<0.001). Multiple pathways demonstrated changes, with arginine/proline ( P=1.2×10-35), glutathione ( P=3.3×10-39), and alanine/aspartate/glutamate ( P=1.4×10-26) metabolism most affected. Six subjects died. Nonsurvivors demonstrated altered aspartate ( P=0.007) and nicotinate/nicotinamide metabolism ( P=0.005). The combination of 24-hour aspartate and methylnicotinamide identified nonsurvivors versus survivors (area under the curve, 0.86; P<0.01), as well as upper/lower 50% intensive care unit length of stay (area under the curve, 0.89; P<0.01). Conclusions The preoperative metabolic fingerprint of neonates differed from older infants. Large metabolic shifts occurred after cardiopulmonary bypass, independent of age. Nonsurvivors and subjects requiring longer intensive care unit length of stay showed distinct changes in metabolism. Specific metabolites, including aspartate and methylnicotinamide, may differentiate sicker patients from those experiencing a more benign course.
Keywords: congenital heart disease; critical care; kynurenic acid; metabolite; metabolome; methylnicotinamide; neonate.
Figures










References
-
- Bujak R, Struck‐Lewicka W, Markuszewski MJ, Kaliszan R. Metabolomics for laboratory diagnostics. J Pharm Biomed Anal. 2015;113:108–120. - PubMed
-
- Tomic V, Russwurm S, Moller E, Claus RA, Blaess M, Brunkhorst F, Bruegel M, Bode K, Bloos F, Wippermann J, Wahlers T, Deigner HP, Thiery J, Reinhart K, Bauer M. Transcriptomic and proteomic patterns of systemic inflammation in on‐pump and off‐pump coronary artery bypass grafting. Circulation. 2005;112:2912–2920. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

