Prescription of Antibacterial Drugs for HIV-Exposed, Uninfected Infants, Malawi, 2004-2010
- PMID: 30561313
- PMCID: PMC6302572
- DOI: 10.3201/eid2501.180782
Prescription of Antibacterial Drugs for HIV-Exposed, Uninfected Infants, Malawi, 2004-2010
Abstract
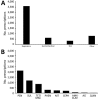
Antimicrobial drug resistance is a serious health hazard driven by overuse. Administration of antimicrobial drugs to HIV-exposed, uninfected infants, a population that is growing and at high risk for infection, is poorly studied. We therefore analyzed factors associated with antibacterial drug administration to HIV-exposed, uninfected infants during their first year of life. Our study population was 2,152 HIV-exposed, uninfected infants enrolled in the Breastfeeding, Antiretrovirals and Nutrition study in Lilongwe, Malawi, during 2004-2010. All infants were breastfed through 28 weeks of age. Antibacterial drugs were prescribed frequently (to 80% of infants), and most (67%) of the 5,329 prescriptions were for respiratory indications. Most commonly prescribed were penicillins (43%) and sulfonamides (23%). Factors associated with lower hazard for antibacterial drug prescription included receipt of cotrimoxazole preventive therapy, receipt of antiretroviral drugs, and increased age. Thus, cotrimoxazole preventive therapy may lead to fewer prescriptions for antibacterial drugs for these infants.
Keywords: HIV-1; HIV/AIDS; Malawi; antibacterial; antibiotic prophylaxis; antibiotic stewardship; antimicrobial resistance; drug resistance; infant; infectious disease medicine; penicillins; poverty; trimethoprim/sulfamethoxazole drug combination.
Figures

References
-
- World Health Organization. Antimicrobial resistance: global report on surveillance. Geneva. Organization. 2014;•••:69–71.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

