Controlled Human Malaria Infection of Healthy Adults With Lifelong Malaria Exposure to Assess Safety, Immunogenicity, and Efficacy of the Asexual Blood Stage Malaria Vaccine Candidate GMZ2
- PMID: 30561539
- PMCID: PMC6763635
- DOI: 10.1093/cid/ciy1087
Controlled Human Malaria Infection of Healthy Adults With Lifelong Malaria Exposure to Assess Safety, Immunogenicity, and Efficacy of the Asexual Blood Stage Malaria Vaccine Candidate GMZ2
Abstract
Background: GMZ2 is a recombinant malaria vaccine inducing immune responses against Plasmodium falciparum (Pf) merozoite surface protein-3 and glutamate-rich protein. We used standardized controlled human malaria infection (CHMI) to assess the efficacy of this asexual blood-stage vaccine.
Methods: We vaccinated 50 healthy, adult volunteers with lifelong exposure to Pf 3 times, at 4-week intervals, with 30 or 100 µg GMZ2 formulated in CAF01, a liposome-based adjuvant; 100 µg GMZ2, formulated in Alhydrogel; or a control vaccine (Verorab). Approximately 13 weeks after the last vaccination, 35/50 volunteers underwent CHMI by direct venous inoculation of 3200 Pf sporozoites (Sanaria® PfSPZ Challenge).
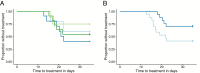
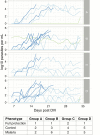
Results: Adverse events were similarly distributed between GMZ2 and control vaccinees. Baseline-corrected anti-GMZ2 antibody concentrations 4 weeks after the last vaccination were higher in all 3 GMZ2-vaccinated arms, compared to the control group. All GMZ2 formulations induced similar antibody levels. CHMI resulted in 29/34 (85%) volunteers with Pf parasitemia and 15/34 (44%) with malaria (parasitemia and symptoms). The proportion of participants with malaria (2/5 control, 6/10 GMZ2-Alhydrogel, 2/8 30 µg GMZ2-CAF01, and 5/11 100 µg GMZ2-CAF01) and the time it took them to develop malaria were similar in all groups. Baseline, vaccine-specific antibody concentrations were associated with protection against malaria.
Conclusions: GMZ2 is well tolerated and immunogenic in lifelong-Pf-exposed adults from Gabon, with similar antibody responses regardless of formulation. CHMI showed no protective effect of prior vaccination with GMZ2, although baseline, vaccine-specific antibody concentrations were associated with protection. CHMI with the PfSPZ Challenge is a potent new tool to validate asexual, blood-stage malaria vaccines in Africa.
Clinical trials registration: Pan-African Clinical Trials: PACTR201503001038304.
Keywords: Plasmodium falciparum; clinical trial; controlled human malaria infection; malaria vaccine.
© The Author(s) 2018. Published by Oxford University Press for the Infectious Diseases Society of America. All rights reserved. For permissions, e-mail: journals.permissions@oup.com.
Figures


References
-
- RTS,S Clinical Trials Partnership. Efficacy and safety of RTS,S/AS01 malaria vaccine with or without a booster dose in infants and children in Africa: final results of a phase 3, individually randomised, controlled trial. Lancet 2015; 386: 31–45. Available at:http://apps.who.int/iris/bitstream/handle/10665/259874/WER9303.pdf. - PMC - PubMed
-
- World Health Organization. Weekly Epidemiological Record 2018; 93:17–9.
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

