IGNITE4: Results of a Phase 3, Randomized, Multicenter, Prospective Trial of Eravacycline vs Meropenem in the Treatment of Complicated Intraabdominal Infections
- PMID: 30561562
- PMCID: PMC6735687
- DOI: 10.1093/cid/ciy1029
IGNITE4: Results of a Phase 3, Randomized, Multicenter, Prospective Trial of Eravacycline vs Meropenem in the Treatment of Complicated Intraabdominal Infections
Abstract
Background: Increasing antimicrobial resistance among pathogens that cause complicated intraabdominal infections (cIAIs) supports the development of new antimicrobials. Eravacycline, a novel member of the fluorocycline family, is active against multidrug-resistant bacteria including extended-spectrum β-lactamase (ESBL) and carbapenem-resistant Enterobacteriaceae.
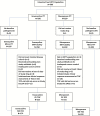
Methods: IGNITE4 was a prospective, randomized, double-blind trial. Hospitalized patients with cIAI received either eravacycline 1 mg/kg every 12 hours or meropenem 1 g every 8 hours intravenously for 4-14 days. The primary objective was to demonstrate statistical noninferiority (NI) in clinical cure rates at the test-of-cure visit (25-31 days from start of therapy) in the microbiological intent-to-treat population using a NI margin of 12.5%. Microbiological outcomes and safety were also evaluated.
Results: Eravacycline was noninferior to meropenem in the primary endpoint (177/195 [90.8%] vs 187/205 [91.2%]; difference, -0.5%; 95% confidence interval [CI], -6.3 to 5.3), exceeding the prespecified margin. Secondary endpoints included clinical cure rates in the modified ITT population (231/250 [92.4%] vs 228/249 [91.6%]; difference, 0.8; 95% CI, -4.1, 5.8) and the clinically evaluable population (218/225 [96.9%] vs 222/231 [96.1%]; (difference, 0.8; 95% CI -2.9, 4.5). In patients with ESBL-producing Enterobacteriaceae, clinical cure rates were 87.5% (14/16) and 84.6% (11/13) in the eravacycline and meropenem groups, respectively. Eravacycline had relatively low rates of adverse events for a drug of this class, with less than 5%, 4%, and 3% of patients experiencing nausea, vomiting, and diarrhea, respectively.
Conclusions: Treatment with eravacycline was noninferior to meropenem in adult patients with cIAI, including infections caused by resistant pathogens.
Clinical trials registration: NCT01844856.
Keywords: Enterobacteriaceae; complicated intraabdominal infection; eravacycline; gram-negative bacteria; multidrug resistance.
© The Author(s) 2018. Published by Oxford University Press for the Infectious Diseases Society of America.
Figures
Comment in
-
Reply to Tang and Lai.Clin Infect Dis. 2020 Jun 10;70(12):2751-2752. doi: 10.1093/cid/ciz856. Clin Infect Dis. 2020. PMID: 31504319 No abstract available.
-
The Safety of Eravacycline in the Treatment of Acute Bacterial Infection.Clin Infect Dis. 2020 Jun 10;70(12):2750-2751. doi: 10.1093/cid/ciz855. Clin Infect Dis. 2020. PMID: 31504330 No abstract available.
References
-
- Antimicrobial resistance threats in the United States, 2013 2013. Available at: https://www.cdc.gov/drugresistance/pdf/ar-threats-2013–508.pdf. Accessed 14 November 2018.
-
- Antimicrobial resistance 2018. Available at: https://ecdc.europa.eu/en/antimicrobial-resistance. Accessed 14 November 2018.
-
- Antimicrobial resistance 2018. Available at: https://www.idsociety.org/public-health/antimicrobial-resistance/antimic.... Accessed 14 November 2018.
-
- Antimicrobial resistance 2018. Available at: http://www.who.int/antimicrobial-resistance/en/. Accessed 14 November 2018.
-
- Chong Y, Shimoda S, Shimono N. Current epidemiology, genetic evolution and clinical impact of extended-spectrum β-lactamase-producing Escherichia coli and Klebsiella pneumoniae. Infect Genet Evol 2018; 61:185–8. - PubMed