PREVAIL I Cluster Vaccination Study With rVSVΔG-ZEBOV-GP as Part of a Public Health Response in Liberia
- PMID: 30561672
- PMCID: PMC6562162
- DOI: 10.1093/infdis/jiy698
PREVAIL I Cluster Vaccination Study With rVSVΔG-ZEBOV-GP as Part of a Public Health Response in Liberia
Abstract
Objective: In November 2015, a 15-year-old boy received a diagnosis of Ebola virus disease (EVD) at the John F. Kennedy Medical Center in Monrovia, Liberia. Two additional family members received a diagnosis of EVD. The protocol for a phase 2 placebo-controlled trial of 2 Ebola vaccines was amended and approved; in 4 days, a single-arm cluster vaccination trial using the Merck rVSVΔG-ZEBOV-GP vaccine was initiated. Here, we evaluate the safety and immunogenicity of the vaccine and discuss challenges for its implementation in a small Ebola outbreak.
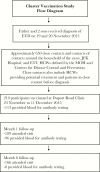
Method: We conducted a ring vaccination study among contacts and contacts of close contacts of EVD cases a in Monrovia. Participants were evaluated 1 and 6 months after vaccination.
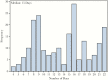
Results: Among 650 close contacts and contacts of close contacts of EVD cases, 210 (32%) consented and were vaccinated with rVSVΔG-ZEBOV-GP. Of those vaccinated, 189 (90%) attended the month 1 follow-up visit; 166 (79%) attended the month 6 visit. No serious adverse events were reported. Among 88 participants without an elevated antibody level at baseline, 77.3% (95% confidence interval, 68.5-86.1) had an antibody response at 1 month.
Conclusions: The Merck rVSVΔG-ZEBOV-GP vaccine appeared to be safe and immunogenic among the vaccinated individuals. However, fewer than one third of eligible individuals consented to vaccination. These data may help guide implementation decisions for of cluster vaccination programs in an Ebola cluster outbreak response situation.
Keywords: Ebola virus; Ebola virus disease; Liberia; PREVAIL; cluster vaccination; outbreak response; rVSVΔG-ZEBOV-GP vaccine; ring vaccination; vaccine.
Published by Oxford University Press for the Infectious Diseases Society of America 2018.
Figures


References
-
- Henao-Restrepo AM, Longini IM, Egger M, et al. . Efficacy and effectiveness of an rVSV-vectored vaccine expressing Ebola surface glycoprotein: interim results from the Guinea ring vaccination cluster-randomised trial. Lancet 2015; 386:857–66. - PubMed
-
- Dokubo EK, Wendland A, Mate SE, et al. Persistence of Ebola virus after the end of widespread transmission in Liberia: an outbreak report. Lancet Infect Dis 2018; 18(9):1015–24. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

