Seamless Designs: Current Practice and Considerations for Early-Phase Drug Development in Oncology
- PMID: 30561713
- PMCID: PMC6376915
- DOI: 10.1093/jnci/djy196
Seamless Designs: Current Practice and Considerations for Early-Phase Drug Development in Oncology
Abstract
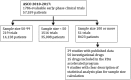
Traditionally, drug development has evaluated dose, safety, activity, and comparative benefit in a sequence of phases using trial designs and endpoints specifically devised for each phase. Innovations in drug development seek to consolidate the phases and rapidly expand accrual with "seamless" trial designs. Although consolidation and rapid accrual may yield efficiencies, widespread use of seamless first-in-human (FiH) trials without careful consideration of objectives, statistical analysis plans, or trial oversight raises concerns. A working group formed by the National Cancer Institute convened to consider and discuss opportunities and challenges for such trials as well as encourage responsible use of these designs. We reviewed all abstracts presented at American Society of Clinical Oncology annual meetings from 2010 to 2017 for FiH trials enrolling at least 100 patients. We identified 1786 early-phase trials enrolling 57 559 adult patients. Fifty-one of the trials (2.9%) investigated 50 investigational new drugs, were seamless, and accounted for 14.6% of the total patients. The seamless trials included a median of 3 (range = 1-13) expansion cohorts. The overall risk of clinically significant treatment-related adverse events (grade 3-4) was 49.1% (range = 0.0-100%), and seven studies reported at least one toxic death. Rapid expansion of FiH trials may lead to earlier drug approval and corresponding widespread patient access to active therapeutics. Nevertheless, seamless designs must adhere to established ethical, scientific, and statistical standards. Protocols should include prospectively planned analyses of efficacy in disease- or biomarker-defined cohorts of sufficient rigor to support accelerated approval.
© The Author(s) 2018. Published by Oxford University Press.
Figures
References
-
- Thomas DW, Burns J, Audette J, Carroll A, Dow-Hygelund C, Hay M. Clinical development success rates 2006–2015. https://www.bio.org/sites/default/files/Clinical%20Development%20Success.... Published June 2016. Accessed December 8, 2017.
-
- DiMasi JA, Grabowski HG.. Economics of new oncology drug development. J Clin Oncol. 2007;252:209–216. - PubMed
-
- Prowell TM, Theoret MR, Pazdur R.. Seamless oncology-drug development. N Engl J Med. 2016;37421:2001–2003. - PubMed
-
- Mullard A. Reining in the supersized phase I cancer trial. Nat Rev Drug Discov. 2016;156:371–373. - PubMed