Polydopamine/Transferrin Hybrid Nanoparticles for Targeted Cell-Killing
- PMID: 30562983
- PMCID: PMC6315732
- DOI: 10.3390/nano8121065
Polydopamine/Transferrin Hybrid Nanoparticles for Targeted Cell-Killing
Abstract
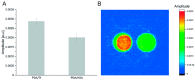
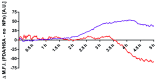
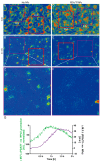
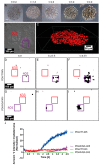
Polydopamine can form biocompatible particles that convert light into heat. Recently, a protocol has been optimized to synthesize polydopamine/protein hybrid nanoparticles that retain the biological function of proteins, and combine it with the stimuli-induced heat generation of polydopamine. We have utilized this novel system to form polydopamine particles, containing transferrin (PDA/Tf). Mouse melanoma cells, which strongly express the transferrin receptor, were exposed to PDA/Tf nanoparticles (NPs) and, subsequently, were irradiated with a UV laser. The cell death rate was monitored in real-time. When irradiated, the melanoma cells exposed to PDA/Tf NPs underwent apoptosis, faster than the control cells, pointing towards the ability of PDA/Tf to mediate UV-light-induced cell death. The system was also validated in an organotypic, 3D-printed tumor spheroid model, comprising mouse melanoma cells, and the exposure and subsequent irradiation with UV-light, yielded similar results to the 2D cell culture. The process of apoptosis was found to be targeted and mediated by the lysosomal membrane permeabilization. Therefore, the herein presented polydopamine/protein NPs constitute a versatile and stable system for cancer cell-targeting and photothermal apoptosis induction.
Keywords: 3D cell printing; cell targeting; live cell imaging; lysosomal membrane permeabilization; polydopamine/transferrin nanoparticles; spheroids; targeted apoptosis in vitro.
Conflict of interest statement
The authors declare no conflict of interest and the funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures




References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

