Synthesis, Biological Evaluation and Low-Toxic Formulation Development of Glycosylated Paclitaxel Prodrugs
- PMID: 30563132
- PMCID: PMC6321537
- DOI: 10.3390/molecules23123211
Synthesis, Biological Evaluation and Low-Toxic Formulation Development of Glycosylated Paclitaxel Prodrugs
Abstract
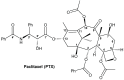
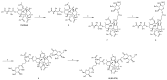
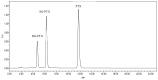
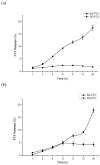
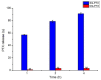
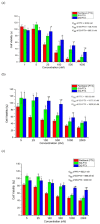
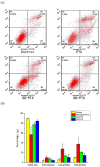
Paclitaxel (PTX) is a famous anti-cancer drug with poor aqueous solubility. In clinical practices, Cremophor EL (polyethoxylated castor oil), a toxic surfactant, is used for dissolution of PTX, which accounts for serious side effects. In the present study, a single glucose-conjugated PTX prodrug (SG-PTX) and a double glucose-conjugated PTX prodrug (DG-PTX) were synthesized with a glycosylated strategy via succinate linkers. Both of the two prodrugs presented significant solubility improvement and drug-like lipophilicities. Compared to DG-PTX, SG-PTX manifested more promising release of the parent drug in serum. A high percentage of PTX released from SG-PTX could be detected after enzymatic hydrolysis of β-glucuronidase. Besides, both of the two prodrugs exhibited effective cytotoxicity against breast cancer cells and ovarian cancer cells, but presented reduced cytotoxicity against normal breast cells. Moreover, SG-PTX manifested impressive solubility in a low toxic formulation (without ethanol) with a different percentage of Cremophor EL. These results indicated that glycosylation is a promising strategy for PTX modification and SG-PTX may be a feasible and potential type of PTX prodrug. In addition, ethanol-free formulation with a low percentage of Cremophor EL might have the potential to develop a safer formulation for further studies of glycosylated PTX prodrugs.
Keywords: anti-cancer; paclitaxel; prodrug; solubility; toxic surfactant.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Well-Defined Redox-Sensitive Polyethene Glycol-Paclitaxel Prodrug Conjugate for Tumor-Specific Delivery of Paclitaxel Using Octreotide for Tumor Targeting.Mol Pharm. 2015 Aug 3;12(8):3020-31. doi: 10.1021/acs.molpharmaceut.5b00280. Epub 2015 Jun 30. Mol Pharm. 2015. PMID: 26086430
-
Nanoparticles Containing High Loads of Paclitaxel-Silicate Prodrugs: Formulation, Drug Release, and Anticancer Efficacy.Mol Pharm. 2015 Dec 7;12(12):4329-35. doi: 10.1021/acs.molpharmaceut.5b00530. Epub 2015 Nov 4. Mol Pharm. 2015. PMID: 26505116 Free PMC article.
-
Synthesis and evaluation of water-soluble poly(vinyl alcohol)-paclitaxel conjugate as a macromolecular prodrug.Biol Pharm Bull. 2008 May;31(5):963-9. doi: 10.1248/bpb.31.963. Biol Pharm Bull. 2008. PMID: 18451527
-
Recent Development of Copolymeric Nano-Drug Delivery System for Paclitaxel.Anticancer Agents Med Chem. 2020;20(18):2169-2189. doi: 10.2174/1871520620666200719001038. Anticancer Agents Med Chem. 2020. PMID: 32682385 Review.
-
Alternative formulations of paclitaxel.Cancer Treat Rev. 1997 Mar;23(2):87-95. doi: 10.1016/s0305-7372(97)90022-0. Cancer Treat Rev. 1997. PMID: 9225960 Review.
Cited by
-
Different Targeting Ligands-Mediated Drug Delivery Systems for Tumor Therapy.Pharmaceutics. 2024 Feb 7;16(2):248. doi: 10.3390/pharmaceutics16020248. Pharmaceutics. 2024. PMID: 38399302 Free PMC article. Review.
-
Natural Taxanes: From Plant Composition to Human Pharmacology and Toxicity.Int J Mol Sci. 2022 Dec 9;23(24):15619. doi: 10.3390/ijms232415619. Int J Mol Sci. 2022. PMID: 36555256 Free PMC article. Review.
-
Novel insights into paclitaxel's role on tumor-associated macrophages in enhancing PD-1 blockade in breast cancer treatment.J Immunother Cancer. 2024 Jul 15;12(7):e008864. doi: 10.1136/jitc-2024-008864. J Immunother Cancer. 2024. PMID: 39009452 Free PMC article.
-
A Gastric Cancer Peptide GX1-Modified Nano-Lipid Carriers Encapsulating Paclitaxel: Design and Evaluation of Anti-Tumor Activity.Drug Des Devel Ther. 2020 Jun 12;14:2355-2370. doi: 10.2147/DDDT.S233023. eCollection 2020. Drug Des Devel Ther. 2020. PMID: 32606603 Free PMC article.
-
Stereoselective Conversions of Carbohydrate Anomeric Hydroxyl Group in Basic and Neutral Conditions.Molecules. 2024 Dec 31;30(1):120. doi: 10.3390/molecules30010120. Molecules. 2024. PMID: 39795176 Free PMC article. Review.
References
-
- Nicolaou K.C., Dai W., Guy R.K. Chemistry and biology of TAXOL. Ange. Chem. Int. Ed. 1994;33:15–44. doi: 10.1002/anie.199400151. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

