The Application of ATR-FTIR Spectroscopy and the Reversible DNA Conformation as a Sensor to Test the Effectiveness of Platinum(II) Anticancer Drugs
- PMID: 30563229
- PMCID: PMC6308638
- DOI: 10.3390/s18124297
The Application of ATR-FTIR Spectroscopy and the Reversible DNA Conformation as a Sensor to Test the Effectiveness of Platinum(II) Anticancer Drugs
Abstract
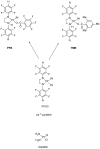
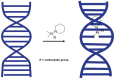
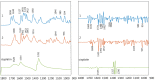
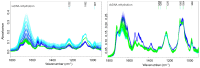
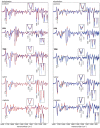
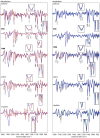
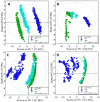
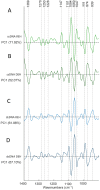
Platinum(II) complexes have been found to be effective against cancer cells. Cisplatin curbs cell replication by interacting with the deoxyribonucleic acid (DNA), reducing cell proliferation and eventually leading to cell death. In order to investigate the ability of platinum complexes to affect cancer cells, two examples from the class of polyfluorophenylorganoamidoplatinum(II) complexes were synthesised and tested on isolated DNA. The two compounds trans-[N,N'-bis(2,3,5,6-tetrafluorophenyl)ethane-1,2-diaminato(1-)](2,3,4,5,6-pentafluorobenzoato)(pyridine)platinum(II) (PFB) and trans-[N,N'-bis(2,3,5,6-tetrafluorophenyl)ethane-1,2-diaminato(1-)](2,4,6-trimethylbenzoato)(pyridine)platinum(II) (TMB) were compared with cisplatin through their reaction with DNA. Attenuated Total Reflection Fourier Transform Infrared (ATR-FTIR) spectroscopy was applied to analyse the interaction of the Pt(II) complexes with DNA in the hydrated, dehydrated and rehydrated states. These were compared with control DNA in acetone/water (PFB, TMB) and isotonic saline (cisplatin) under the same conditions. Principle Component Analysis (PCA) was applied to compare the ATR-FTIR spectra of the untreated control DNA with spectra of PFB and TMB treated DNA samples. Disruptions in the conformation of DNA treated with the Pt(II) complexes upon rehydration were mainly observed by monitoring the position of the IR-band around 1711 cm-1 assigned to the DNA base-stacking vibration. Furthermore, other intensity changes in the phosphodiester bands of DNA at ~1234 cm-1 and 1225 cm-1 and shifts in the dianionic phosphodiester vibration at 966 cm-1 were observed. The isolated double stranded DNA (dsDNA) or single stranded DNA (ssDNA) showed different structural changes when incubated with the studied compounds. PCA confirmed PFB had the most dramatic effect by denaturing both dsDNA and ssDNA. Both compounds, along with cisplatin, induced changes in DNA bands at 1711, 1088, 1051 and 966 cm-1 indicative of DNA conformation changes. The ability to monitor conformational change with infrared spectroscopy paves the way for a sensor to screen for new anticancer therapeutic agents.
Keywords: DNA; IR; platinum.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
Similar articles
-
ATR-FTIR spectroscopy shows changes in ovarian cancer cells after incubation with novel organoamidoplatinum(ii) complexes.Analyst. 2018 Dec 3;143(24):6087-6094. doi: 10.1039/c8an01558a. Analyst. 2018. PMID: 30457585
-
Novel platinum-based anticancer drug: a complete vibrational study.Acta Crystallogr C Struct Chem. 2018 May 1;74(Pt 5):628-634. doi: 10.1107/S2053229618005843. Epub 2018 Apr 23. Acta Crystallogr C Struct Chem. 2018. PMID: 29726474
-
Binding of platinum derivative, oxaliplatin to deoxyribonucleic acid: structural insight into antitumor action.J Biomol Struct Dyn. 2019 Sep;37(14):3838-3847. doi: 10.1080/07391102.2018.1531059. Epub 2018 Dec 5. J Biomol Struct Dyn. 2019. PMID: 30282523
-
The importance of hydration and DNA conformation in interpreting infrared spectra of cells and tissues.Chem Soc Rev. 2016 Apr 7;45(7):1980-98. doi: 10.1039/c5cs00511f. Chem Soc Rev. 2016. PMID: 26403652 Review.
-
Cisplatin and platinum drugs at the molecular level. (Review).Oncol Rep. 2003 Nov-Dec;10(6):1663-82. Oncol Rep. 2003. PMID: 14534679 Review.
Cited by
-
Novel quercetin and apigenin-acetamide derivatives: design, synthesis, characterization, biological evaluation and molecular docking studies.RSC Adv. 2020 Jun 30;10(42):25046-25058. doi: 10.1039/d0ra04559d. eCollection 2020 Jun 29. RSC Adv. 2020. PMID: 35517443 Free PMC article.
-
Molecular Spectroscopic Markers of DNA Damage.Molecules. 2020 Jan 28;25(3):561. doi: 10.3390/molecules25030561. Molecules. 2020. PMID: 32012927 Free PMC article. Review.
-
Synergistic effects of silver nanoparticles and cisplatin in combating inflammation and hyperplasia of airway stents.Bioact Mater. 2021 Jul 29;9:266-280. doi: 10.1016/j.bioactmat.2021.07.029. eCollection 2022 Mar. Bioact Mater. 2021. PMID: 34820570 Free PMC article.
-
Detection of Human Cholangiocarcinoma Markers in Serum Using Infrared Spectroscopy.Cancers (Basel). 2021 Oct 12;13(20):5109. doi: 10.3390/cancers13205109. Cancers (Basel). 2021. PMID: 34680259 Free PMC article.
-
Cell Phase Identification in a Three-Dimensional Engineered Tumor Model by Infrared Spectroscopic Imaging.Anal Chem. 2023 Feb 14;95(6):3349-3357. doi: 10.1021/acs.analchem.2c04554. Epub 2022 Dec 27. Anal Chem. 2023. PMID: 36574385 Free PMC article.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

