Mechanisms of Matrix-Induced Chemoresistance of Breast Cancer Cells-Deciphering Novel Potential Targets for a Cell Sensitization
- PMID: 30563275
- PMCID: PMC6315379
- DOI: 10.3390/cancers10120495
Mechanisms of Matrix-Induced Chemoresistance of Breast Cancer Cells-Deciphering Novel Potential Targets for a Cell Sensitization
Abstract
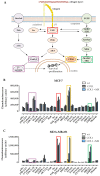
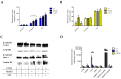
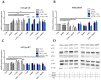
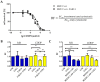
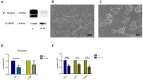
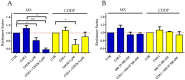
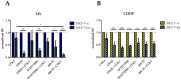
Tumor cell binding to microenvironment components such as collagen type 1 (COL1) attenuates the sensitivity to cytotoxic drugs like cisplatin (CDDP) or mitoxantrone (MX), referred to as cell adhesion mediated drug resistance (CAM-DR). CAM-DR is considered as the onset for resistance mutations, but underlying mechanisms remain elusive. To evaluate CAM-DR as target for sensitization strategies, we analyzed signaling pathways in human estrogen-positive MCF-7 and triple-negative MDA-MB-231 breast cancer cells by western blot, proteome profiler array and TOP-flash assay in presence of COL1. β1-Integrins, known to bind COL1, appear as key for mediating COL1-related resistance in both cell lines that primarily follows FAK/PI3K/AKT pathway in MCF-7, and MAPK pathway in MDA-MB-231 cells. Notably, pCREB is highly elevated in both cell lines. Consequently, blocking these pathways sensitizes the cells evidently to CDDP and MX treatment. Wnt signaling is not relevant in this context. A β1-integrin knockdown of MCF-7 cells (MCF-7-β1-kd) reveals a signaling shift from FAK/PI3K/AKT to MAPK pathway, thus CREB emerges as a promising primary target for sensitization in MDA-MB-231, and secondary target in MCF-7 cells. Concluding, we provide evidence for importance of CAM-DR in breast cancer cells and identify intracellular signaling pathways as targets to sensitize cells for cytotoxicity treatment regimes.
Keywords: CAM-DR; CREB; FAK; MAPK; Wnt signaling; breast cancer; cisplatin; collagen; integrin; mitoxantrone.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
β1-Integrin binding to collagen type 1 transmits breast cancer cells into chemoresistance by activating ABC efflux transporters.Biochim Biophys Acta Mol Cell Res. 2020 May;1867(5):118663. doi: 10.1016/j.bbamcr.2020.118663. Epub 2020 Jan 25. Biochim Biophys Acta Mol Cell Res. 2020. PMID: 31987794
-
Targeting Discoidin Domain Receptor 1 (DDR1) Signaling and Its Crosstalk with β1-integrin Emerges as a Key Factor for Breast Cancer Chemosensitization upon Collagen Type 1 Binding.Int J Mol Sci. 2020 Jul 13;21(14):4956. doi: 10.3390/ijms21144956. Int J Mol Sci. 2020. PMID: 32668815 Free PMC article.
-
The Impact of Integrin-Mediated Matrix Adhesion on Cisplatin Resistance of W1 Ovarian Cancer Cells.Biomolecules. 2019 Nov 26;9(12):788. doi: 10.3390/biom9120788. Biomolecules. 2019. PMID: 31779287 Free PMC article.
-
Insight into Cisplatin-Resistance Signaling of W1 Ovarian Cancer Cells Emerges mTOR and HSP27 as Targets for Sensitization Strategies.Int J Mol Sci. 2020 Dec 3;21(23):9240. doi: 10.3390/ijms21239240. Int J Mol Sci. 2020. PMID: 33287446 Free PMC article.
-
CAM-DR: Mechanisms, Roles and Clinical Application in Tumors.Front Cell Dev Biol. 2021 Jul 6;9:698047. doi: 10.3389/fcell.2021.698047. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34295898 Free PMC article. Review.
Cited by
-
Comprehensive analysis of prognostic significance of cadherin (CDH) gene family in breast cancer.Aging (Albany NY). 2022 Oct 30;14(20):8498-8567. doi: 10.18632/aging.204357. Aging (Albany NY). 2022. PMID: 36315446 Free PMC article.
-
Extracellular Matrix in the Tumor Microenvironment and Its Impact on Cancer Therapy.Front Mol Biosci. 2020 Jan 31;6:160. doi: 10.3389/fmolb.2019.00160. eCollection 2019. Front Mol Biosci. 2020. PMID: 32118030 Free PMC article. Review.
-
α2β1 Integrin specific inhibitor BTT-3033 promotes paclitaxel-induced apoptosis in human ovarian cancer cells.Res Pharm Sci. 2024 Oct 22;19(5):549-560. doi: 10.4103/RPS.RPS_245_23. eCollection 2024 Oct. Res Pharm Sci. 2024. PMID: 39691300 Free PMC article.
-
Cancer cells grown in 3D under fluid flow exhibit an aggressive phenotype and reduced responsiveness to the anti-cancer treatment doxorubicin.Sci Rep. 2020 Jul 21;10(1):12020. doi: 10.1038/s41598-020-68999-9. Sci Rep. 2020. PMID: 32694700 Free PMC article.
-
A novel approach for engineering DHCM/GelMA microgels: application in hepatocellular carcinoma cell encapsulation and chemoresistance research.Front Bioeng Biotechnol. 2025 Mar 14;13:1564543. doi: 10.3389/fbioe.2025.1564543. eCollection 2025. Front Bioeng Biotechnol. 2025. PMID: 40161518 Free PMC article.
References
-
- Zhu L.-C., Gao J., Hu Z.-H., Schwab C.L., Zhuang H.-Y., Tan M.-Z., Yan L.-M., Liu J.-J., Zhang D.-Y., Lin B. Membranous expressions of Lewis y and CAM-DR-related markers are independent factors of chemotherapy resistance and poor prognosis in epithelial ovarian cancer. Am. J. Cancer Res. 2015;5:830–843. - PMC - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

