Drosophila p53 integrates the antagonism between autophagy and apoptosis in response to stress
- PMID: 30563404
- PMCID: PMC6526837
- DOI: 10.1080/15548627.2018.1558001
Drosophila p53 integrates the antagonism between autophagy and apoptosis in response to stress
Abstract
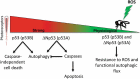
The tumor suppressor TP53/p53 is a known regulator of apoptosis and macroautophagy/autophagy. However, the molecular mechanism by which TP53 regulates 2 apparently incompatible processes remains unknown. We found that Drosophila lacking p53 displayed impaired autophagic flux, higher caspase activation and mortality in response to oxidative stress compared with wild-type flies. Moreover, autophagy and apoptosis were differentially regulated by the p53 (p53B) and ΔNp53 (p53A) isoforms: while the former induced autophagy in differentiated neurons, which protected against cell death, the latter inhibited autophagy by activating the caspases Dronc, Drice, and Dcp-1. Our results demonstrate that the differential use of p53 isoforms combined with the antagonism between apoptosis and autophagy ensures the generation of an appropriate p53 biological response to stress.
Keywords: Apoptosis; Parkinson disease; caspase; macroautophagy; neurodegenerative disease; oxidative stress; p53; photoreceptor.
Figures






References
-
- Vousden KH, Lane DP.. p53 in health and disease. Nat Rev Mol Cell Biol. 2007. April;8(4):275–283. PubMed PMID: 17380161. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
