The endoplasmic reticulum (ER) chaperone BiP is a master regulator of ER functions: Getting by with a little help from ERdj friends
- PMID: 30563838
- PMCID: PMC6369273
- DOI: 10.1074/jbc.REV118.002804
The endoplasmic reticulum (ER) chaperone BiP is a master regulator of ER functions: Getting by with a little help from ERdj friends
Abstract
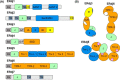
The endoplasmic reticulum (ER) represents the entry point into the secretory pathway where nascent proteins encounter a specialized environment for their folding and maturation. Inherent to these processes is a dedicated quality-control system that detects proteins that fail to mature properly and targets them for cytosolic degradation. An imbalance in protein folding and degradation can result in the accumulation of unfolded proteins in the ER, resulting in the activation of a signaling cascade that restores proper homeostasis in this organelle. The ER heat shock protein 70 (Hsp70) family member BiP is an ATP-dependent chaperone that plays a critical role in these processes. BiP interacts with specific ER-localized DnaJ family members (ERdjs), which stimulate BiP's ATP-dependent substrate interactions, with several ERdjs also binding directly to unfolded protein clients. Recent structural and biochemical studies have provided detailed insights into the allosteric regulation of client binding by BiP and have enhanced our understanding of how specific ERdjs enable BiP to perform its many functions in the ER. In this review, we discuss how BiP's functional cycle and interactions with ERdjs enable it to regulate protein homeostasis in the ER and ensure protein quality control.
Keywords: ATPase; BiP; ER-localized DnaJ proteins; GRP78; endoplasmic reticulum (ER); endoplasmic reticulum–associated protein degradation (ERAD); heat shock protein (HSP); molecular chaperone; protein folding; protein misfolding; stress response; unfolded protein response (UPR).
© 2019 Pobre et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures

References
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

