Transcriptional Profiling of Patient Isolates Identifies a Novel TOR/Starvation Regulatory Pathway in Cryptococcal Virulence
- PMID: 30563896
- PMCID: PMC6299223
- DOI: 10.1128/mBio.02353-18
Transcriptional Profiling of Patient Isolates Identifies a Novel TOR/Starvation Regulatory Pathway in Cryptococcal Virulence
Abstract
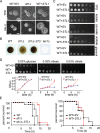
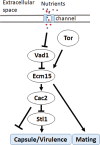
Human infection with Cryptococcus causes up to a quarter of a million AIDS-related deaths annually and is the most common cause of nonviral meningitis in the United States. As an opportunistic fungal pathogen, Cryptococcus neoformans is distinguished by its ability to adapt to diverse host environments, including plants, amoebae, and mammals. In the present study, comparative transcriptomics of the fungus within human cerebrospinal fluid identified expression profiles representative of low-nutrient adaptive responses. Transcriptomics of fungal isolates from a cohort of HIV/AIDS patients identified high expression levels of an alternative carbon nutrient transporter gene, STL1, to be associated with poor early fungicidal activity, an important clinical prognostic marker. Mouse modeling and pathway analysis demonstrated a role for STL1 in mammalian pathogenesis and revealed that STL1 expression is regulated by a novel multigene regulatory mechanism involving the CAC2 subunit of the chromatin assembly complex 1, CAF-1. In this pathway, the global regulator of virulence gene VAD1 was found to transcriptionally regulate a cryptococcal homolog of a cytosolic protein, Ecm15, in turn required for nuclear transport of the Cac2 protein. Derepression of STL1 by the CAC2-containing CAF-1 complex was mediated by Cac2 and modulated binding and suppression of the STL1 enhancer element. Derepression of STL1 resulted in enhanced survival and growth of the fungus in the presence of low-nutrient, alternative carbon sources, facilitating virulence in mice. This study underscores the utility of ex vivo expression profiling of fungal clinical isolates and provides fundamental genetic understanding of saprophyte adaption to the human host.IMPORTANCECryptococcus is a fungal pathogen that kills an estimated quarter of a million individuals yearly and is the most common cause of nonviral meningitis in the United States. The fungus is carried in about 10% of the adult population and, after reactivation, causes disease in a wide variety of immunosuppressed individuals, including the HIV infected and patients receiving transplant conditioning, cancer therapy, or corticosteroid therapy for autoimmune diseases. The fungus is widely carried in the soil but can also cause infections in plants and mammals. However, the mechanisms for this widespread ability to infect a variety of hosts are poorly understood. The present study identified adaptation to low nutrients as a key property that allows the fungus to inhabit these diverse environments. Further studies identified a nutrient transporter gene, STL1, to be upregulated under low nutrients and to be associated with early fungicidal activity, a marker of poor clinical outcome in a cohort of HIV/AIDS patients. Understanding molecular mechanisms involved in adaptation to the human host may help to design better methods of control and treatment of widely dispersed fungal pathogens such as Cryptococcus.
Keywords: CAC2; CAF-1; Cryptococcus neoformans; STL1; TOR pathway; VAD1.
Figures




References
-
- Jarvis JN, Bicanic T, Loyse A, Namarika D, Jackson A, Nussbaum JC, Longley N, Muzoora C, Phulusa J, Taseera K, Kanyembe C, Wilson D, Hosseinipour MC, Brouwer AE, Limmathurotsakul D, White N, van der Horst C, Wood R, Meintjes G, Bradley J, Jaffar S, Harrison T. 2014. Determinants of mortality in a combined cohort of 501 patients with HIV-associated cryptococcal meningitis: implications for improving outcomes. Clin Infect Dis 58:736–745. doi:10.1093/cid/cit794. - DOI - PMC - PubMed
-
- Beardsley J, Wolbers M, Kibengo FM, Ggayi AB, Kamali A, Cuc NT, Binh TQ, Chau NV, Farrar J, Merson L, Phuong L, Thwaites G, Van Kinh N, Thuy PT, Chierakul W, Siriboon S, Thiansukhon E, Onsanit S, Supphamongkholchaikul W, Chan AK, Heyderman R, Mwinjiwa E, van Oosterhout JJ, Imran D, Basri H, Mayxay M, Dance D, Phimmasone P, Rattanavong S, Lalloo DG, Day JN, CryptoDex Investigators. 2016. Adjunctive dexamethasone in HIV-associated cryptococcal meningitis. N Engl J Med 374:542–554. doi:10.1056/NEJMoa1509024. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
